1. Background
Nanotechnology has great potential in the treatment of bacterial infections. Metal nanoparticles can enhance the effects of marketed drugs and/or target bacteria as an alternative to antibiotics (1). For example, nanoparticles have been used as antibacterial coatings to prevent infections (2). Drugs conjugated with nanoparticles are considered as efficient drug delivery systems that suffer from poor bioavailability for enhanced activity to treat infections, while some nanoparticles have also been used for bacterial detection (2).
Silver in particular is found extraordinarily effective against several microbes (3). Its strong antimicrobial properties, chemical inertness and high thermal stability offer potential applications (3). Although silver ions possess strong antimicrobial properties, they are easily sequestered by chloride, phosphate and proteins. Silver nanoparticles are less susceptible to being intercepted and offer an effective delivery mechanism. Hence, the nanoparticle form is used preferably to ferry silver ions to bacteria for effective killing. To this end, several lines of evidence suggest that silver nanoparticles (AgNPs) exhibit potent antibacterial properties against Escherichia coli by release of silver ions (4).
Several derivatives of thiopyridine are promising candidates for killing growing and dormant bacterial cells (5, 6). In bacteria, transition metals such as copper, along with iron and manganese are used as a catalyzer for electron transfer reactions in metallo-enzymes such as cytochrome oxidase (7). Copper is a vital cofactor of various enzymes, albeit free copper is highly toxic to living cells. Bacteria have developed specific copper homeostasis systems that commonly act as defense mechanisms to maintain the cellular metabolism at ambient copper concentrations (8). In Gram-negative bacteria, the inner membrane pumps heavy metal (P1B-type ATPases) transferring cytoplasmic copper to periplasm. Hence, transition metal homeostasis and detoxification are crucial factors for cell viability (9). Recently, metal ions such as Cu(II) and Hg(II) are shown to exert synergistic effects on the antibacterial activity of AgNPs (10, 11).
2. Objectives
Since copper species are known to affect the permeability of the outer membrane of E. coli (12), the overall aim of this study was to determine whether conjugation with AgNPs enhances antibacterial effects of thiopyridine. We further determined whether Cu(I) enhanced antibacterial activity of thiopyridine-conjugated silver nanoparticles against E. coli.
3. Methods
3.1. Chemical and Reagents
All chemicals and reagents were purchased from Sigma-Aldrich, unless otherwise stated. Escherichia coli was obtained from the American type culture collection (ATCC 8739). Deionized water was used for the synthesis and analysis of ThPy-AgNPs. UV-visible spectra were recorded at thermo scientific evolution 300 spectrophotometer and atomic force microscopy (AFM) analysis was carried out by the ACAFM mode on Agilent technologies 5500 AFM instrument.
3.2. Synthesis of ThPy-AgNPs
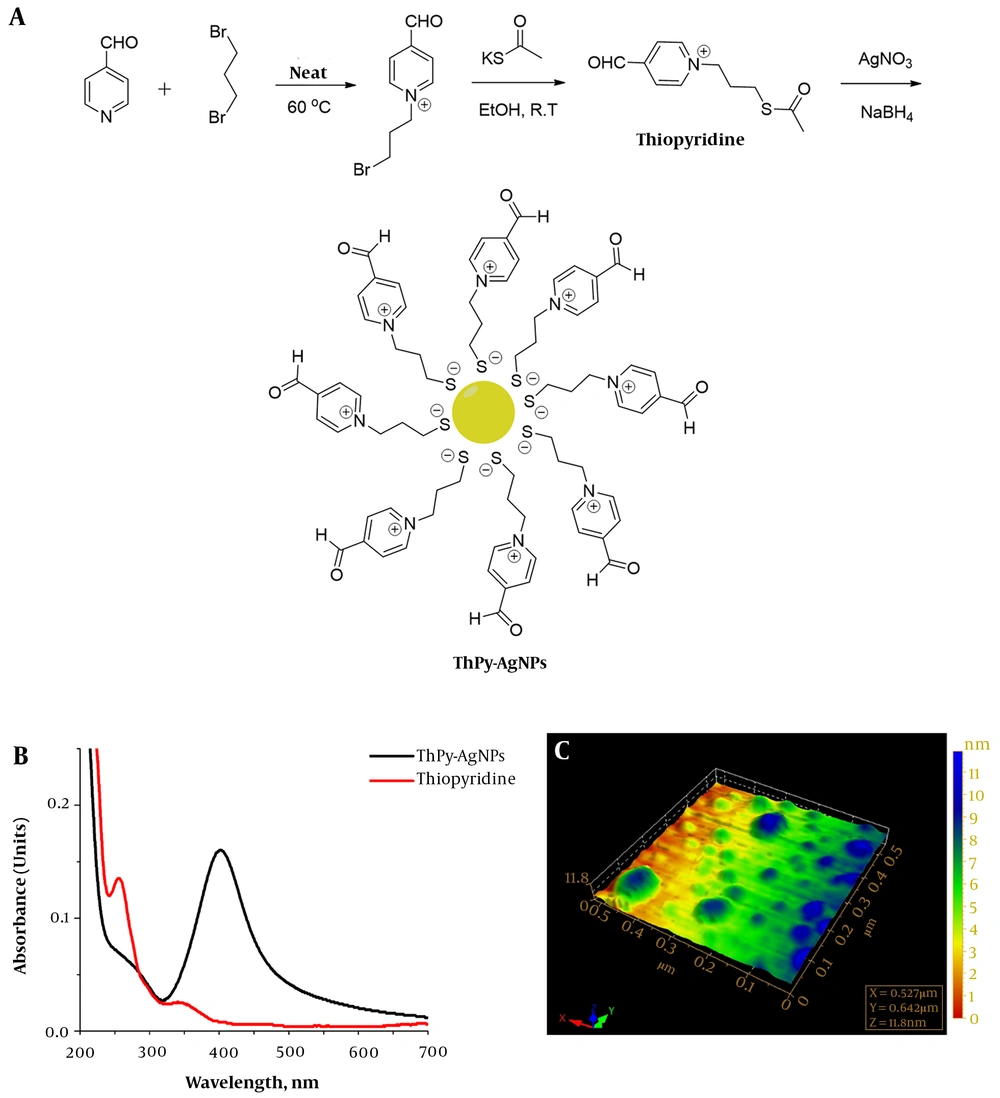
Thiopyridine and ThPy-AgNPs were synthesized as described previously (13). Briefly, 0.9 mL 4-formylpyridine (1 mmol) was stirred in 3 mL dibromopropane as a solvent. The solution was magnetically stirred and heated at 60°C for 8 hours. The brown precipitates formed after 8 hours were filtered to remove excess solvent. The precipitates were washed by chloroform to remove unreacted formyl pyridine, followed by washing with methanol to obtain pure N-alkylated product in the viscous gummy liquid form resulting from evaporation of methanol. Next, 229 mg of N-alkylated product (1 mmol) was dissolved in 8 mL of ethanol, and 137 mg potassium thioacetate (1.2 mmol) was added to the stirring solution at room temperature.
The progress of the reaction was monitored using thin layer chromatography (dichloromethane: Methanol, 9:1). After completion of reaction (around 10 hours), ethanol was evaporated at reduced pressure to obtain a brown solid crude product. The crude product was subsequently washed with chloroform followed by methanol. 1-(3-(Acetylthio) propyl)-4-formylpyridin-1-ium (thiopyridine) was obtained from the methanol washing in brown crystalline state after slow evaporation of the solvent. Thiopyridine was characterized by Fourier transformation infrared spectroscopy, electrospray mass spectrometry and proton nuclear magnetic resonance spectroscopy.
ThPy-AgNPs (1 mM) were prepared as follows; 10:1 (v/v) thiopyridine to AgNO3 aqueous solution was mixed and stirred for 2 hours in the presence of sodium borohydride. The reaction mixture turned yellow-brown from colorless, and the UV-visible spectral profile and AFM analysis were carried out to determine the successful formation of ThPy-coated AgNPs (13).
3.3. Minimum Inhibitory Concentration (MIC)
Agar well diffusion method was employed to calculate MICs. Briefly, MIC was measured for ThPy alone and AgNPs-coated ThPy. Briefly, Mueller Hinton agar was used as medium to develop a lawn of E. coli ATCC 8739 at 106 cells/mL concentration. In some experiments, Mueller Hinton agar containing Cu(I) was prepared. Plates were incubated with ThPy-AgNPs and ThPy alone. Duplicate dilutions were used to calculate minimum zones of inhibition. In each well, various concentrations of ThPy-AgNPs ranging from 5 to 500 µg/mL were added. Plates were incubated for 2 hours at room temperature to facilitate the diffusion process before incubation for 24 - 48 hours at 37°C. The zones of inhibition were measured by using a millimeter scale.
3.4. Cytotoxic Activity of Cu(I) Against Escherichia coli
Freshly harvested E. coli ATCC 8739 cells isolated from tryptone soya agar were seeded at 106 cells in each well of 96-well plate. Next, various concentrations of Cu(I) were serially diluted in Muller Hinton broth, then 200 µL of each concentration was added to duplicate wells and the plate was incubated for 18 - 24 hours at 37°C. Following this incubation, 20 µL of MTT (stock concentration of 5 mg/mL) was added to each well and the plate was incubated at 37°C for 3 hours. Absorbance was recorded at 570 nm with the reference wavelength of 650 nm (14).
3.5. Inductively Coupled Plasma (ICP-OES) Spectroscopy
To examine the permeability of nanoparticles loaded with Cu(I), different doses of ThPy-AgNPs and copper (I) were incubated with E. coli (106 CFU/mL) at 37°C for 24 hours in two sets. After incubation, bacterial colonies were enumerated by plating on nutrient agar plates for one set. For the second set, bacteria were centrifuged at 12000 × g for 1 hour at 4°C. The supernatants were discarded while the resultant pellet was incubated with 10 mL of 1% nitric acid for the digestion of the samples. The digested suspension was filtered using a 0.45-µm syringe filter and analyzed by ICP-OES with standards of silver and copper, and untreated E. coli were used as controls (15).
3.6. Atomic Force Microscopy Imaging
Escherichia coli ATCC 8739 was cultured on tryptic soy agar (Oxoid, UK) at 37°C for 24 hours. Ten µL of each test sample was applied onto a poly-L-lysine (PLL)-coated glass slide and allowed to dry at room temperature. Meanwhile, freshly incubated culture was diluted to make 106 CFU of E. coli in distilled water, and 10 µL of this solution was casted onto a freshly cleaved mica surface and dried at room temperature. Next, the samples were rinsed with Milli-Q water and air-dried at room temperature. The samples were then subjected to AFM for morphological analysis. High frequency 125 µm length Si cantilever was used with force constant of 42 N/m and resonance frequency of 330 kHz.
4. Results
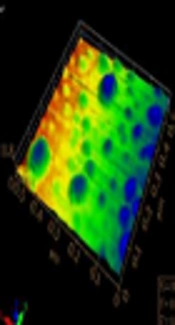
Formyl pyridine reaction with dibromopropane was controlled to obtain monosubstituted product by using excessive dibromopropane (as solvent, instead of stoichiometric ratio). The obtained salt N-alkylated product was found to be an ionic liquid after removal of solvents. In the next step, bromo group was substituted by thioacetate nucleophile in polar protic solvent (Figure 1A). The optimized reaction conditions suited the formation of thiopyridine favorably as implied by high-yield and purity. Thiopyridine was finally used to stabilize silver nanoparticles synthesized by one phase reduction by sodium borohydride (13). Figure 1B describes the typical surface plasmon resonance band of the AgNPs for ThPy-conjugated AgNPs with absorption maxima at 402 nm, while 1C shows the size and morphology of spherical polydispersed nanoconjugates with the average diameter of 60 nm.
To evaluate the antibacterial effects of silver nanoparticles conjugation with thiopyridine against E. coli, inhibition zone assay was performed. Since ThPy-AgNPs were found to display specific affinity towards Cu(I) ions and copper homeostasis plays a pivotal role in the defense mechanism of E. coli, we aimed to test the effects of intracellular interactions of Cu(I) and ThPy-AgNPs on their antibacterial potency. Bacteria were treated with multiple doses of ThPy-AgNPs, which upon conjugating with Cu(I) ions damaged the cellular walls and disrupted the morphology of the microbe (Table 1 and Figure 3). The safe Cu(I) accumulation dosage for E. coli ATCC 8739 is found to be less than 40 µg/mL in which the cells can digest the Cu(I) to regulate the metabolic system. The MICs were calculated for thiopyridine and ThPy-AgNPs with and without Cu(I) ions by using the inhibition zone method as mentioned in the Methods section. Minimum inhibitory concentration of thiopyridine is calculated to be 200 µg/mL, whereas for ThPy-AgNPs it is obtained at 100 µg/mL.
| Concentration, µg/mL | Zones of Inhibition, ThPy Alone, mm | Zones of Inhibition, ThPy Alone, with Cu(I), mm | Zones of Inhibition, ThPy-AgNPs, mm |
|---|---|---|---|
| 5 | - | - | - |
| 10 | - | - | - |
| 25 | - | - | - |
| 50 | 10 ± 0.1 | 10.1 ± 0.3 | 12 ± 0.2 |
| 100 | 12 ± 0.2 | 12.3 ± 0.2 | 15 ± 0.2 |
| 200 | 15 ± 0.3 | 15.2 ± 0.2 | 17 ± 0.3 |
| 300 | 19 ± 0.2 | 19.2 ± 0.3 | 20 ± 0.4 |
| 400 | 21 ± 0.4 | 21.1 ± 0.3 | 23 ± 0.5 |
| 500 | 23 ± 0.3 | 23 ± 0.2 | 26 ± 0.3 |
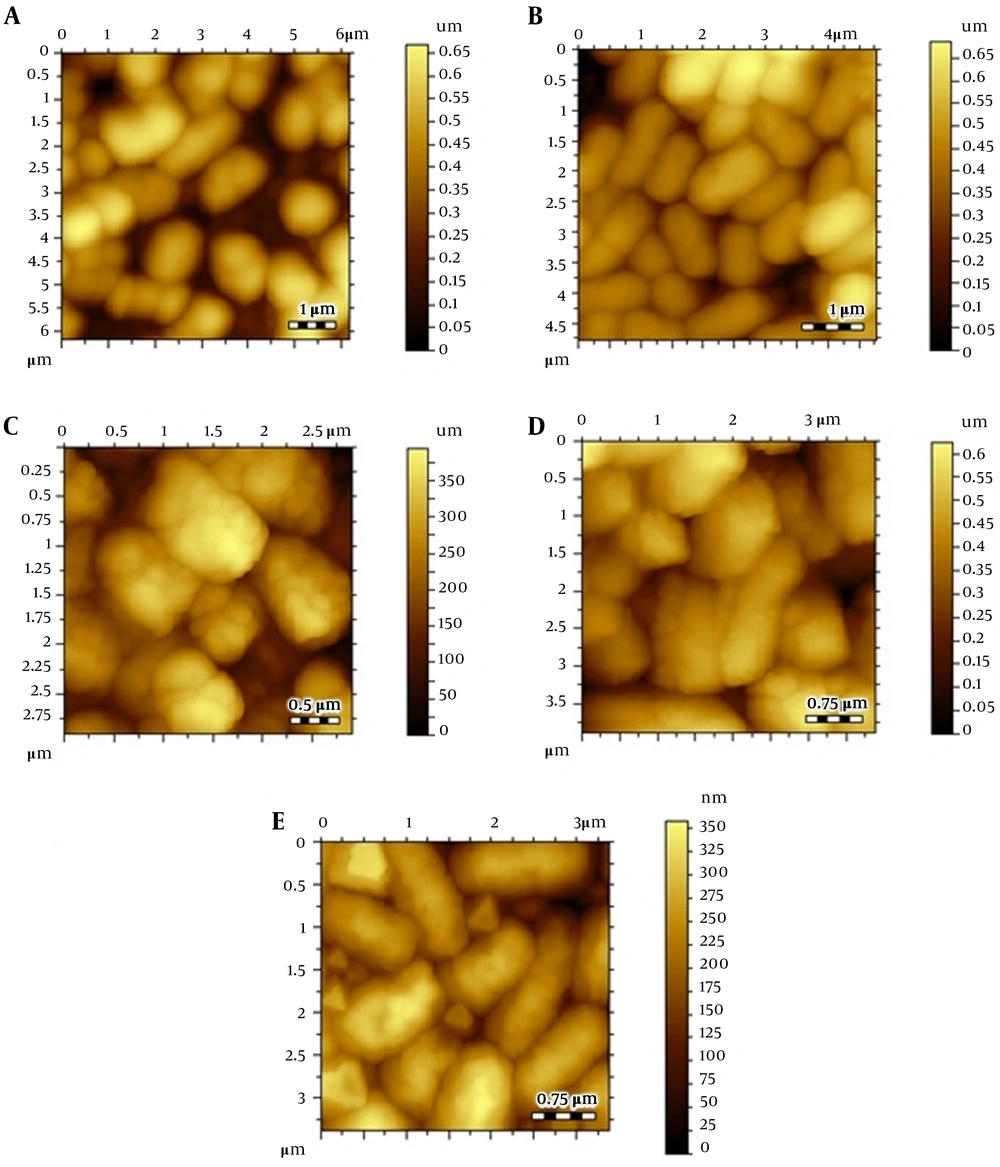
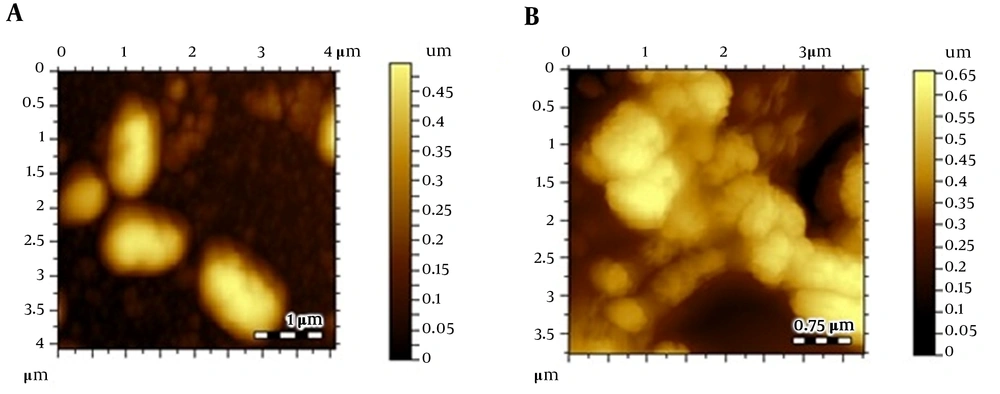
Atomic force microscopic images; (A) Escherichia coli control, (B) E. coli after treating Cu(I), (C) thiopyridine-treated E. coli at 4 hours, (D) thiopyridine-treated E. coli at 8 hours, and (E) thiopyridine-treated E. coli pretreated with Cu(I) at 4 hours. The results are representative of at least three independent experiments.
Inductively coupled plasma (ICP) spectroscopy analysis (Table 2) showed that E. coli contained 0.167 ppm Cu(I) in blank samples, which strengthens our hypothesis that copper is used in the metabolism of bacteria. Different Cu(I) amounts were incubated with E. coli, and it can be clearly observed that at 30 to 40 ppm of test samples, the amount entering the cells of the sample increased suggesting the effective interaction of ThPy-AgNPs and Cu(I). However, at concentrations higher than 40 ppm of Cu(I), it was toxic and started to kill the bacteria. Therefore, the number of nanoparticles entering the cells declined, which further dropped down to only 1.582 ppm at 100 ppm test nanoparticles. While at 100 ppm in the absence of Cu(I), only 0.009 ppm nanoparticles were infused in the cells.
| Tests Concentration | Copper Concentration by ICP, ppm | Silver Concentration by ICP, ppm |
|---|---|---|
| Blank | 0.169 | 0.0 |
| Blank | 0.166 | 0.0 |
| 1 | 0.614 | 0.628 |
| 5 | 2.021 | 1.690 |
| 10 | 2.215 | 1.973 |
| 30 | 2.975 | 2.053 |
| 40 | 5.364 | 4.631 |
| 80 | 5.364 | 4.474 with 40 ppm Cu(I) |
| 100 | - | 1.582 with 40 ppm Cu(I) |
| 100 | - | 0.009 without Cu(I) |
Atomic force microscopy images of control E. coli showed a smooth cell surface with no ruptures or indentations as displayed in Figure 2A. Figure 2B depicts the image of E. coli treated with 30 µg/mL (below the MIC) Cu(I), and it is clearly evident that Cu(I)-treated cells are healthier and contain smoother surface as compared to control cells. This is due to the fact that at non-cytotoxic dosage of Cu(I) ions, these ions are used in the metabolism of E. coli.
Escherichia coli cultures were also treated with thiopyridine alone (MIC, 200 µg/mL), and cell morphology was examined via AFM with variable incubation times. The images revealed a roughness in the surface after 4 hours (Figure 2C - E), while the cellular degradation increased with time, and after 8 hours, the cells were degraded. Since the unconjugated AgNPs demonstrated poor antibacterial efficiency as reported in our previous study (16), their role as cytotoxic agents against E. coli was ruled out. Furthermore, to balance the effect of Cu(I)-mediated infusion of nanoparticles in E. coli, we incubated Cu(I)-pretreated cells with thiopyridine, which showed no difference in cell morphology as compared to ligand used alone. Hence, AFM analysis was found to be in complete agreement with the MIC data. ThPy-AgNPs were found to destroy E. coli more effectively (MIC, 100 µg/mL) compared to thiopyridine alone (MIC, 200 µg/mL) within 4 hours (Figure 3).
5. Discussion
Here, we synthesized, characterized, and tested thiopyridine ligand and ThPy-AgNPs against E. coli ATCC 8739. MICs were calculated by the zone of inhibition method, while AFM provided the detailed morphological events occurred at the cellular level. The membrane of E. coli is made up of inhomogeneous packing of lipopolysaccharide (LPS); therefore, it is a bit rough at nanoscale (17). The packing of the adjacent LPS patches is quite tight, which produces a permeable barrier (17, 18). Since metal ions play a key role in safeguarding the assembly of the LPS and membrane proteins within the outer membrane (19), these can be exploited to increase cell permeability. The use of metal ions to attain the liberation of the periplasmic substances has been presented in several literature reports (20).
Since the mode of action of nanoparticles depends on their physicochemical properties, particularly their surface features, it was observed that ThPy-AgNPs can easily penetrate the bacterial cells when pre-treated with Cu(I) by adhering to proteins in the cell membrane due to selective supramolecular interaction of ThPy-AgNPs with Cu(I) ions. On the other hand, unconjugated AgNPs and thiopyridine were found to be less effective in disrupting the cell membrane as shown in Table 1. Reports on the mechanism of antimicrobial actions of silver ions suggest that upon silver ions treatment, DNA regulation and expression of ribosomal subunit proteins alter while some other cellular proteins and enzymes producing ATP are inactivated (4). It has also been suggested that silver ions primarily disturb the function of membrane-bound enzymes, for example as in the respiratory chain (3, 4).
For thiopyridine and ThPy-AgNPs, AFM images were acquired from MIC samples and only relatively intact cells were observed. Therefore, these images represent only those cells which showed minimal cell lysis at a particular sample concentration. A logical trend is observed in the roughness of cell membrane, which increased with enhanced sample concentration, and ThPy-AgNPs had the most drastic effect on the surface roughness as suggested by the MIC data.
5.1. Conclusions
In sum, we demonstrated antibacterial action of a novel thiopyridine ligand in comparison with AgNPs after capping with it. The antibacterial activity of Cu(I)-doped ThPy-AgNPs was studied against E. coli ATCC 8739. The quantitative measurements by conventional antibacterial assays, the use of AFM to determine surface integrity, and morphological alterations indicated that ThPy-AgNPs were twice as effective as free thiopyridine ligand in E. coli lysis. The lower concentration of Cu(I) maximized cell permeability of ThPy-AgNPs without exhibiting cytotoxicity. This work clearly demonstrates that integrating nanotechnology and microbiology can lead to possible advancement in the formulation of new types of antibacterial agents.