1. Background
Toxoplasmosis, caused by a protozoan parasite Toxoplasma gondii, is one of the most common parasitic infections among humans and other warm-blooded animals, which can cause serious complications in immunodeficient patients (1, 2). Although immunocompetent individuals with preliminary toxoplasmosis are often asymptomatic, and latent infection can persist for a long time of the host life (3), in immunosuppressed patients, particularly patients with AIDS, this parasite can reactivate and cause more complications, usually when the CD4+ count falls below 100 cells/µL (4, 5). Toxoplasmosis is the most common central nervous system infection in patients diagnosed with AIDS and cerebral toxoplasmosis is the most common cerebral focal lesion in the patients, chiefly among those who have not received the appropriate prophylaxis.
Worldwide prevalence rate of latent toxoplasmosis in HIV-infected patients, including Latin America, Europe, Asia, Africa, and United States have been reported a range of prevalence estimates of 11% - 80%. Hitherto, remarkable progress has been made in immunological and molecular techniques for the diagnosis of T. gondii infection. Choosing suitable diagnostic methods and their elucidation may differ for each clinical immunocompetent and or immunodeficient patients. In immunocompromised patients chronically infected by T. gondii who have positive T. gondii specific IgG antibody prior to the immunosuppression onset, additional serologic testing adds very little to the diagnostic evaluation or it may be misleading (6, 7). In these patients, increasing IgG and IgM titers stating evident reactivation can be indicated in the absence of clinical infections. Therefore, in regards to additional diagnostic methods for immunosuppressed patients suspected to have toxoplasmosis is essential. These methods include PCR amplification to detect T. gondii DNA in various clinical specimens including blood (6, 8), amniotic fluid (9, 10), cerebrospinal fluid (11), and tissue biopsy (12).
2. Objectives
Recently, numerous studies have reported toxoplasmosis on human and animal hosts in the study area, however, there is no sufficient data available regarding toxoplasmosis in HIV+ patients (8, 11). Therefore, in this study we evaluated the prevalence of T. gondii in the blood of HIV-Infected patients from the city of Ahvaz, southwest Iran, by serological (ELISA) and molecular techniques (nested PCR). The aim of this study was to evaluate the utility of ELISA IgG-avidity test for detecting T. gondii infections among HIV-infected patients and comparing immunological methods with nested PCR as molecular assays in the diagnosis of T. gondii.
3. Methods
3.1. Ethics Statement
This research was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (ethics code: 181 1817202862).
3.2. Patients and Blood Samples
In the present study, the blood samples obtained from 379 AIDS patients who were previously confirmed as HIV infected by Western blot and ELISA tests at Reference Laboratory of Treatment and Health Institute in the city of Ahvaz, southwest Iran, were analyzed. Serum samples and buffy coat were prepared and stored in -20°C until used.
3.3. Immunological Tests
3.3.1. ELISA
Presence of IgM and IgG antibodies in sera were determined by ELISA Toxoplasma kits (Trinity Biotech Toxoplasma gondii IgG and IgM ELISA kit- USA).
3.3.2. IgG Avidity Test
Detection of T. gondii-IgG antibodies in human sera was done and interpreted based on the directions of NovaLisa®Toxoplasma gondii IgG Avidity Test (Toxo-IgG-avidity kit; NOVA TEC- GmbH) through the ELISA system.
3.4. Molecular Tests
3.4.1. Nested PCR
DNA from buffy coat of 379 samples was extracted using a commercial purification system (DynaBio™ Blood/Tissue DNA Extraction Mini Kit) and nested PCR assay was performed on the samples targeting a recently discovered repetitive 194-bp DNA sequence (194 - 200 bp) or B1 gene in this parasite (13). Specific primers for amplification of T. gondii-DNA sequence were respectively:
Forward primer 1: 5’-TCAAGCAGCGTATTGTCGAG- < g > -3’, Nucleotide positions (663 - 682)
Reverse primer 1: 5’-CCGCAGCGACTTCTATCTCT- < c > -3’, Nucleotide positions (949 - 930)
Forward primer 2: 5’-GGAACTGCATCCGTTCATGAG- < g > -3’, Nucleotide positions (694 - 14)
Reverse primer 2: 5’-TCTTTAAAGCGTTCGTGGTC- < c > -3’, Nucleotide positions (887 - 868).
DNA was amplified using thermal cycler (Eppendorf AG 22331, Hamburg, Germany) on the following conditions: 5 minutes at 94°C followed by 30 cycles of 25 seconds at 94°C, 20 seconds at 53°C, 20 seconds at 72°C, and a final elongation at 72°C for 5 minutes. For each sample, one positive control and one negative control were considered. The amplification products were diluted in a ratio of 1:10 with D.D.W and was then prepared for the second-step of nested PCR. Second-step was similar to the first, however, with different primers (F2, R2) and under these conditions: 5 minutes at 94°C followed by 35 cycles of 30 seconds at 94°C, 30 seconds at 51.5°C, 45 seconds at 72°C, and a final elongation at 72°C for 10 minutes. PCR products were segregated on a 2% (w/v) agarose gel and visualized by staining with DNA Safe Stain.
3.4.2. DNA Sequencing of PCR Products
In this study, 11 PCR-positive samples that showed a single high-intensity band, were selected. Then, they were sent to Korea Bioneer Corporation along with their second-step PCR products and primers in order for DNA sequencing, and also to search for sequence similarity (homology) of samples sequence with each other and with DNA sequences available in GenBank Home.
3.5. Data Analysis
Statistical analysis of agreement between ELISA and PCR tests were performed using the Kappa agreement test. Differences between groups were assessed by Chi-square test. Values of P ≤ 0.05 were considered statistically significant.
4. Results
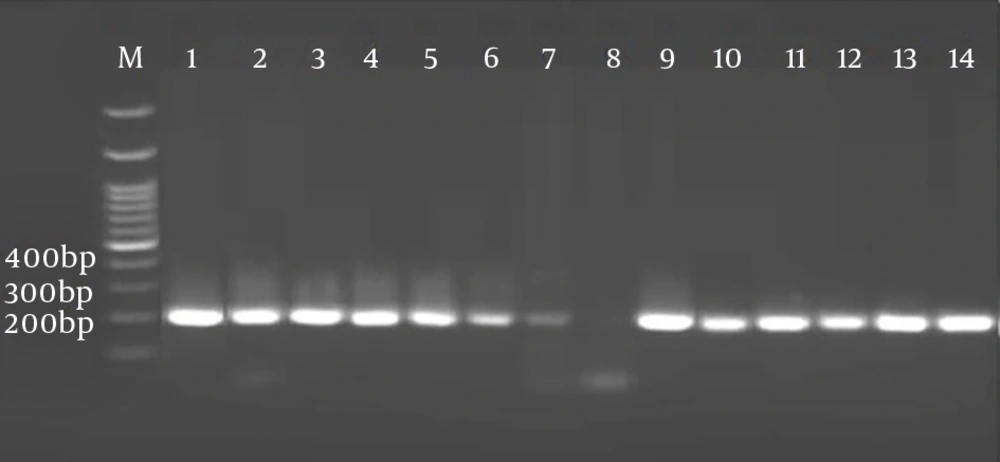
According to ELISA, out of 379 serum samples, 131 (34.56%) and 11 (2.90%) were positive regarding anti-T. gondii IgG and IgM antibodies, respectively. Nested PCR results showed that 64 out of 379 (16.88%) of the samples had DNA molecules of B1 gene (Figure 1). In IgG avidity test, 43 (32.82%) and 88 (67.17%) had antibodies indicating the acute and chronic phase, respectively (Table 1). There was an association between CD4 count and prevalence of toxoplasmosis (P = 0.038). There was almost a perfect agreement (Cohen’s kappa > 0.80) between the results reported by two technicians of ELISA and PCR methods. Genomic sequences of 11 isolates recorded in the World Gene Bank home (LC057644.1 - LC057654.1) had a high homology (more than 98%) with KC607827.1 isolate (Kumar D et al, 2013), based on comparison of Graf obtained from the Clustal W software (Figure 2).
| ELISA | Number | Nested PCRa |
|---|---|---|
| Low IgG avidity (acute) | 43 | 43 (100) |
| High IgG avidity (chronic) | 88 | 9 (10.2) |
| IgM+ and IgG+ | 12 | 12 (100) |
| Only IgM+ | 11 | 11 (100) |
| Seronegative | 237 | 1 (0.4) |
aValues are expressed as No. (%).
5. Discussion
We evaluated 379 HIV infected patients for acute and chronic toxoplasmosis by using anti-Toxoplasma IgG and IgM immunoglobulins and specific B1 gene. The result indicated a relatively high prevalence of acute T. gondii infection in HIV cases and also overlapping the results from two serological and molecular methods. Diagnosis of toxoplasmosis can be conducted by the serological tests, PCR or nested PCR, immounoperoxidase stain, and or isolation of the parasite. Merging of serological tests is often required to determine whether a person has been more possibly infected in the distant past or newly infected. Moreover, an IgM test is used in order to support for determining whether a patient has been recently infected or in the distant past. Due to the potent possibility of misinterpretation as a positive result for IgM test, confirmatory tests should be done. Although there is a wide distribution of the commercial test kits to measure IgM antibodies, these kits often times have low specificity and the misinterpreted reports of the results (14). Moreover, IgM antibodies can remain for several months and continue to more than one year.
Recently, several tests have been introduced for avidity of Toxoplasma IgG antibodies to differentiate between acute and chronic infections. Vidal et al. suggested a combination of immunological and molecular tests for the diagnosis of AIDS-related cerebral toxoplasmosis based on evidences of a study on 64 AIDS patients in Brazil. In the present study, the overlap between serological and molecular techniques in the diagnosis of AIDS-related toxoplasmosis was investigated; the results of our study were confirming Vidal’s study (15).
In this study, 43 (32.82%) HIV-infected patients had Toxoplasma-specific IgG antibodies, suggesting an acute infection warranting appropriate therapeutic intervention. On avidity testing, 88 (67.17%) of 131 IgG -positive patients had high-avidity IgG antibodies indicating a past T. gondii infection. Sensitivity of the ELISA IgG-avidity test is high for detecting a recent T. gondii infection in IgM-positive individuals (16). Such results have been confirmed in the present study by a sensitivity of 100%. In addition, maturation of the IgG response varies considerably between individuals and thus, low-avidity antibodies may persist for months to more than one year (17). In such patients, if an avidity test result was used alone it would have been misinterpreted as suggestive of an acute infection. Several studies have proved that PCR can actually detect T. gondii in the blood of AIDS patients (18, 19); this was also confirmed in our study.
Haghpanah et al. evaluated various clinical stages of toxoplasmosis on immunocompetent patients using the serological and PCR techniques. The PCR result was negative in the chronic phase and primary acute phase. They demonstrated an increase in IgG titer and a reduction in IgM titer during the chronic phase, and low titer of IgM and low or no titer of IgG in the acute phase. Their findings showed that the result of PCR was only positive in patients who were certainly in acute phase (high titers of IgM and low titers of IgG) (20). In our study, from 43 individuals who had acutely low IgG avidity was 43 (100%), while from 88 who had chronically toxoplasmosis phase, nine (10.2%) were detected by PCR method. Only one of the 237 seronegative toxoplasmosis samples yielded a positive result in nested PCR method. These results agree with the study of Haghpanah et al. (20). Parmley et al. reported that DNA detection in cerebrospinal fluid and blood is associated with poor results and variable sensitivity (21). Against this study, we successfully detected the B1 gene of toxoplasma in the blood samples of AIDS infected patients.
Our result showed a statistically significant relationship between patients with CD4+ T < 200 cells/µL and IgG-positive patients (P = 0.038). Studies indicated a relationship between central nervous system (CNS) disease and CD4 cell count < 200 cells/µL (22-24). Individuals with cerebral toxoplasmosis possess higher titers of anti-T. gondii IgG antibodies than patients with other diseases. In a study reported by Eliaszewicz et al. in France, 79% of patients with neurological signs that had CD4 counts < 150 cells/mm3 were displayed (25). In one study by Sucilathangam et al. CD4 counts were < 100 cells/mcl in Toxoplasma seropositive patients (26).
Representing of high titers of anti-T. gondii IgG antibodies, with high IgG avidity, indicates the secondary reactivation of latent or chronic Toxoplasma infection (27). Therefore, it is imperative to detect Toxoplasma seropositivity status in all HIV-infected patients to evaluation the risk for cerebral toxoplasmosis. The result of serology test in the present study is in accordance with the study of Anuradha and Preethi who observed seroprevalence of 34.78% among HIV-positive patients (28). However, there is no agreement with the studies reported by Spalding et al. (74.5%) (29) in south Brazil, Jeannel et al. (63.7%) (30) in Paris, Kodym et al. (30%) (31) in Chezech Republic, and Xiao et al. (12.5%) (32) in China.
5.1. Conclusions
Results of serological techniques (ELISA and IgG avidity) had a higher overlap with nested PCR in identifying T. gondii in seropositive patients. Accordingly, it is suggested that using the IgG-avidity test in condition that PCR testing, is impossible; particularly to distinguish recently acquired infection from past infection.