1. Background
Leishmaniasis is one of major parasitic diseases, which causes various health complications for human populations particularly in endemic regions. The agent responsible for this disease is a protozoan parasite of the Trypanosomatidae family and Leishmania genus. The development of disease depends on many factors, such as the parasite species, inoculum size, and route of transmission, host immunity, and genetic attributes (1-3). Two pentavalent antimonials, such as glucantime (meglumine antimoniate) and pentostam (sodium stibogluconate), are the first line treatment against cutaneous leishmaniasis (4, 5). These drugs have many common side effects, which obscure their therapeutic successes. These include toxicity, drug resistance, refractory wounds, relapse of illness, and more recently, Leishmania-HIV coinfection. Hence, treating the disease is necessary, particularly when humans are the main host (6).
Many studies have examined medical herbs, nanoparticles, and other chemotherapeutic agents to treat leishmaniasis. Cromolyn sodium and ketotifen inhibit penetration of parasites to cells by stabilizing cell membrane. H1 antagonist drugs inhibit mastocyte secretion and release of histamine, chemokine, and other vasoactive substances, known to be involved in asthma and allergic rhinitis. These drugs have also shown anti-stress effects, besides their common anti-allergic properties (7).
Ketotifen, a tricyclic antihistaminic drug (Zaditen®), is prescribed widely as tablets or syrups for treatment of allergic disorders, such as allergic conjunctivitis, chronic rhinitis, urticaria, and asthma. Cromolyn sodium (Allergocrom® and Intal®), prescribed as inhalant capsules, nasal inhalers, and eye drops, is one of the highly consumed and effective antihistamines in the treatment of respiratory sensitivities caused by allergens. Cromolyn sodium inhibits histamine and leukotriene release as well as activation of inflammatory cells, such as eosinophils. However, there is no comprehensive study yet to determine the effectivity of these drugs in the treatment of cutaneous leishmaniasis (8, 9).
2. Objectives
The current study therefore aimed at investigating cytotoxic damage and programmed apoptosis induced by ketotifen and cromolyn sodium to Leishmania major parasites.
3. Methods
3.1. Ethics Statement
This study was approved by the Ethics Committee of Iran University of Medical Sciences with approval Code No:IR.IUMS.REC.FMD. 96-01-30-30371.
3.2. Preparation of Drugs
The pure powders of ketotifen (C19H19NOS) with a molecular weight of 309.426 g/mol and cromolyn sodium (C23H14Na2O11 ) with a molecular weight of 512.334 g/mol were obtained from Sigma-Aldrich. The stock solutions of ketotifen were prepared with distilled water to make a consecrated solution of 250 µg/mL. Desired concentrations were prepared by dilution. glucantime was also purchased from Sigma, and used as a standard control drug. The solutions of glucantime were prepared as mentioned above.
3.3. Parasite Culture
For this study, the Iranian standard strain of Leishmania (MRHO/IR/75/ER) was obtained from Tehran University of Medical Sciences. Batch production of Leishmania parasites was carried out in RPMI 1640 media, which was purchased from Gibco©. As supplementary, 10% Fetal Bovine Serum (FBS) was added to the media as well as 100 unit/mL penicillin and 100 mg/mL streptomycin, to inhibit bacterial growth. The culture was then kept under 18 to 24ºC in an incubator and examined daily under an invert microscope. Cell passage was carried out every 72 hours and each test was done in triplicates.
3.4. Promastigote Growth Inhibition Assay
In order to calculate IC50 of the tested drugs on L. major promastigotes, similar aliquots of promastigotes, containing 106 cell milliliters were added to various concentration (5, 10, 15, 20 µg/mL) of ketotifen and cromolyn sodium drugs at 24, 48, and 72 hours. The temperature was maintained at 18°C to 24°C and test plates were examined under the microscope on a daily basis. One well, containing only promastigote and medium, was designated as the negative control, whereas another containing glucantime with concentration 20 µg/mL and medium in addition to promastigotes was designated as the positive control. Finally, IC50 was obtained by plotting the applied doses against time intervals to produce a dose-response curve and calculate the corresponding linear regression (10).
3.5. The MTT Assay of Anti-Leishmanial Activity of Drugs Against Promastigotes
The colorimetric method of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium (MTT) assay was used to evaluate anti-leishmanial activity of tested drugs. To this end, aliquots containing 106 logarithmic phase promastigotes, were added to different doses of drugs, prepared in 96-well plates under completely sterile conditions. Wells on the edges were filled with PBS to reduce evaporation. The plates were incubated for 24, 48 and 72 hours at 18°C to 24°C. Wells containing medium in each plate were assigned as the negative control, while others containing medium and glucantime were assigned as the positive control. After incubation, plates were taken out, centrifuged, and supernatants were decanted before adding the MTT solution. Plates were again incubated for four hours under the same temperature. This allowed an adequate time for the MTT yellow solution to permeate to parasite cells to produce purple formazan crystals by reacting with succinate dehydrogenase, available in kinetoplasts. To dissolve formazan crystals, dimethyl sulfoxide (DMSO) was then added to the wells and stirred in darkness on a shaker for 10 to 15 minutes. Absorption ratio was read at the 490 nm wavelength, using an enzyme linked immunosorbent assay (ELISA) reader. Given that the number of viable cells correlates with optical density (OD) at the absorbed wavelength, percentages of viable cells in control and test wells were calculated using the corresponding formula (10).
3.6. MTT Assay to Evaluate Drug Toxicity on Macrophages
Balb/c peritoneal macrophages (J774A.1 cell line), provided by the Faculty of Health of Tehran University, were cultured at 105 per well in plates, before adding drug concentrations. The wells on edges were filled with PBS to prevent evaporation in wells containing cells. The MTT solution was added to wells after an incubation period of 24, 48, and 72 hours, followed by incubation for four more hours at 18°C to 24°C. Supernatants were then decanted and DMSO was added to all wells. The absorption was read at 490 nm wavelength, using an ELISA reader. To determine relative absorption (SI: stimulation index), the optical density of each sample was calibrated against the background of absorption in the control well (medium plus macrophage).
3.7. Amastigote Growth Inhibition Assay
Promastigotes in static phase (106 cells) in addition to macrophages and different doses of ketotifen and cromolyn sodium (5, 10, 15, and 20 µg/mL) were added to an eight-well chamber slide. One chamber was filled only with macrophages acting as the negative control and another was filled with medium and glucantime, with concentration of 20 µg/mL, acting as the positive control chamber. The plates were then incubated for 24, 48, and 72 hours at 35°C. The parasites that were not introduced to the macrophage, were entered from the environment by washing with PBS. The prepared smears were then fixed with methanol and stained with Giemsa. Amastigote count in 100 macrophages was done by averaging the number of amastigotes in each macrophage. To obtain the average number of amastigotes in each macrophage, the obtained number was divided by one hundred.
3.8. Cell Death Assessment Using Flow-Cytometry
Annexin V and propidium iodide (PI) staining were applied to differentiate between necrotic and apoptotic promastigotes and amastigotes-infected macrophages, which had been exposed to various doses of ketotifen and cromolyn sodium. For this purpose, cells exposed to various drug doses as well as control cells exposed to PBS were centrifuged for 10 minutes at 1400 g force and then washed. According to the kit manual, 100 µL of Annexin-V solution and PI solution was then added to sedimented cells. Suspension was incubated for 15 minutes at room temperature in a dark place. Color intensity of absorbed Annexin-V by the cells was measured using the FACSCaliber device and results were analyzed with the Cell Quest software (11, 12).
3.9. Statistical Analysis
One-way analysis of variances (One-way ANOVA) to compare the mean of parasite count in each drug and also in its related concentrations and t-test for detecting significant differences between control and test groups were performed to analyze the results using the SPSS software version 20. The difference was considered significant at P < 0.05.
4. Results
4.1. Assessment of Drug Efficacy Using the Parasite Count
After 24, 48, and 72 hours of incubation in the presence of different doses of ketotifen parasite, it was shown that the number of promastigotes was reduced to significant levels compared with the control group (P < 0.05). The IC50 of ketotifen after 72 hours was calculated to be 2.04 µg/mL. IC50 of cromolyn sodium was estimated to be 17.67 µg/mL after 72 hours of exposure. Parasite counts (mean ± SD) upon exposure to 20 µg/mL concentration of glucantime at the same time intervals post-exposure were 0.7 ± 0.05, 0.58 ± 0.07, and 0.47 ± 0.03, and in the control group were 2.15 ± 0.02, 2.25 ± 0.03, and 2.29 ± 0.03 respectively. There were not any significant differences between different concentrations of drugs (P > 0.05). (Tables 1 and 2).
| Ketotifen, µg/mL | Promastigote (106) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 5 | 1.35 ± 0.01 | 1.14 ± 0.02 | 1.10 ± 0.02 |
| 10 | 1.25 ± 0.01 | 0.99 ± 0.02 | 0.80 ± 0.02 |
| 15 | 1.24 ± 0.03 | 1.06 ± 0.04 | 0.77 ± 0.01 |
| 20 | 1.21 ± 0.03 | 1.01 ± 0.03 | 0.71 ± 0.02 |
Number (Mean ± SD) of Promastigotes After Adding Ketotifena
| Cromolyn Sodium, µg/mL | Promastigote (106) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 5 | 1.82 ± 0.02 | 1.61 ± 0.03 | 1.40 ± 0.02 |
| 10 | 1.80 ± 0.03 | 1.68 ± 0.01 | 1.32 ± 0.04 |
| 15 | 1.76 ± 0.03 | 1.57 ± 0.02 | 1.28 ± 0.02 |
| 20 | 1.56 ± 0.02 | 1.36 ± 0.02 | 1.02 ± 0.03 |
Number (Mean ± SD) of Promastigotes After Adding Cromolyn Sodiuma
4.2. Results of MTT Assays for Viable Cells Assessment
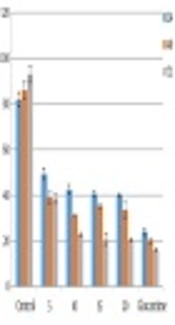
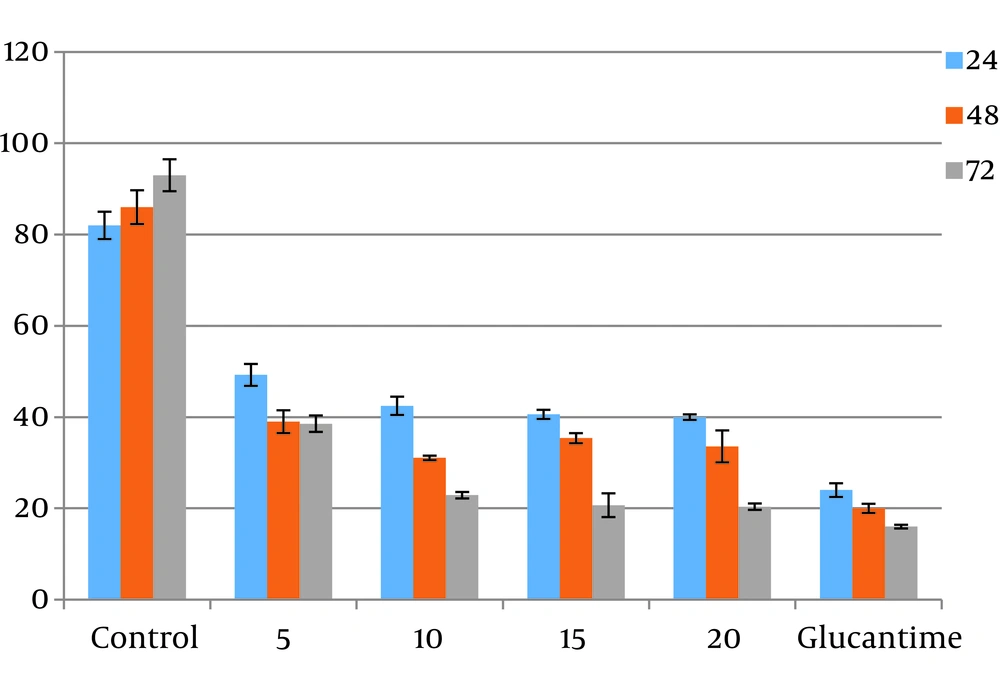
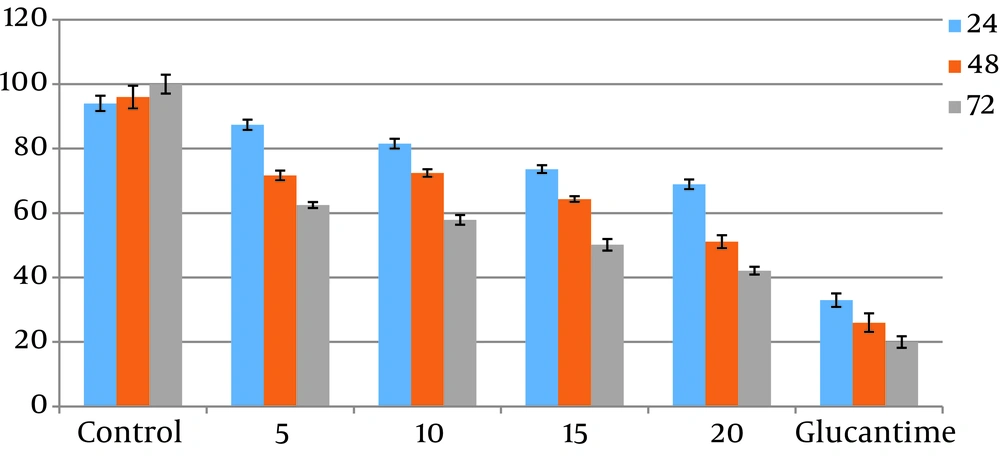
The promastigotes exposed to the highest concentration of ketotifen (20 µg/mL) during 72 hours of incubation showed 20% survival. This result was significantly different from the control test (P < 0.05). Cells incubated with cromolyn sodium at the same concentration and for the same exposure time showed only 35% viability, which also differed significantly from the control (P < 0.05) (Figures 1 and 2). There were not any significant differences between different concentrations of drugs (P > 0.05).
4.3. Results of MTT Assays for Toxicity Assessment
Toxicity of different doses of drugs were assessed on macrophage cells. Results showed higher toxicity than cromolyn sodium (53%) compared to ketotifen (17%). All the results were statistically significant compared to the controls (P < 0.05). These results were the average of at least three separate experiments.
4.4. Number of Amastigotes Inside Macrophage Cells
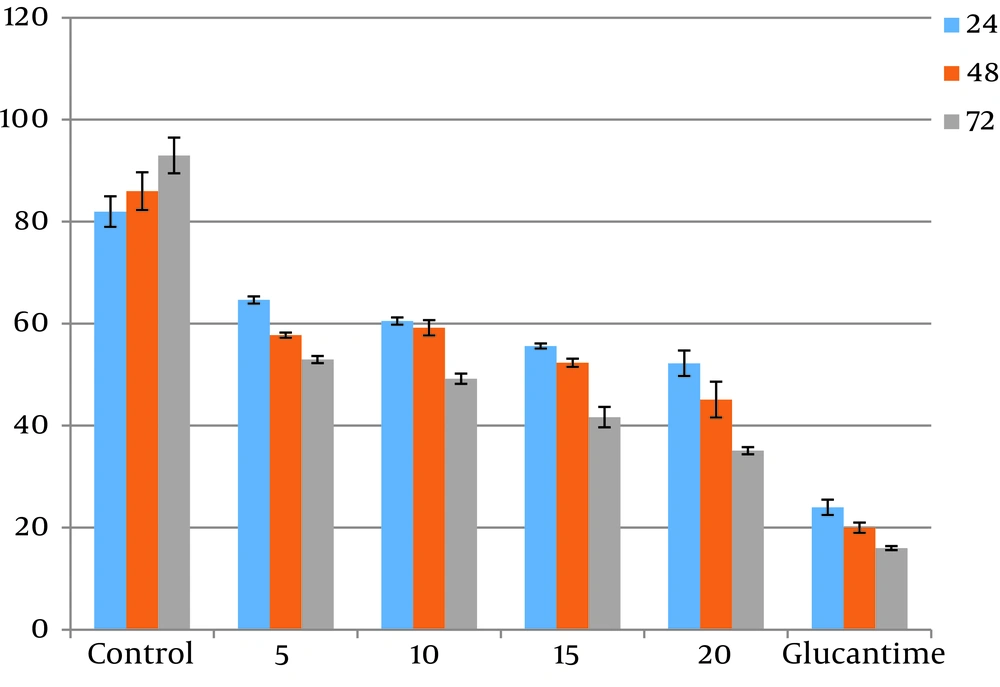
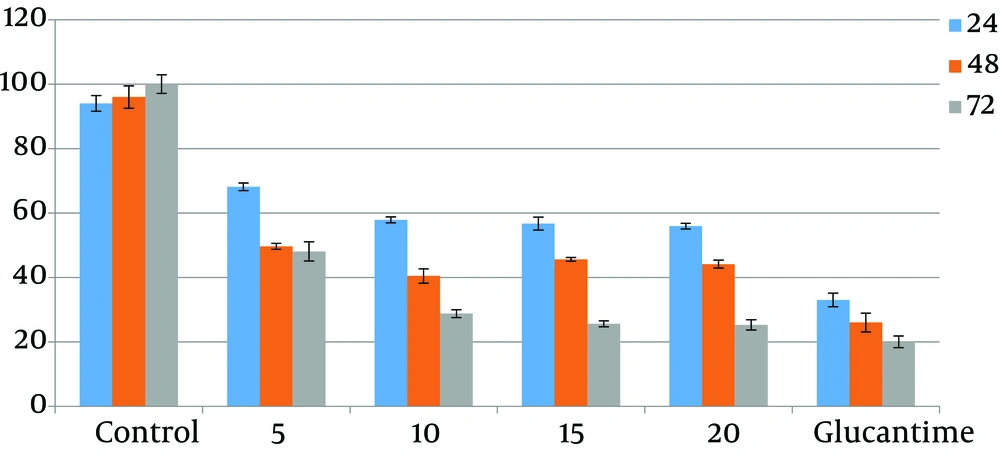
The results acquired from numeration of intracellular amastigotes showed that drugs reduced the viability of amastigotes in J774.A1 macrophages at a concentration and dose-dependent mode. Differences between test and control groups were significant (P < 0.05). IC50 of ketotifen after 72 hours of exposure was calculated to be 0.12 µg/mL while IC50 of cromolyn sodium for the same exposure time was 14.79 µg/mL (Figures 3 and 4). There were not any significant differences between different concentrations of drugs (P > 0.05).
4.5. Results of Flow-Cytometry
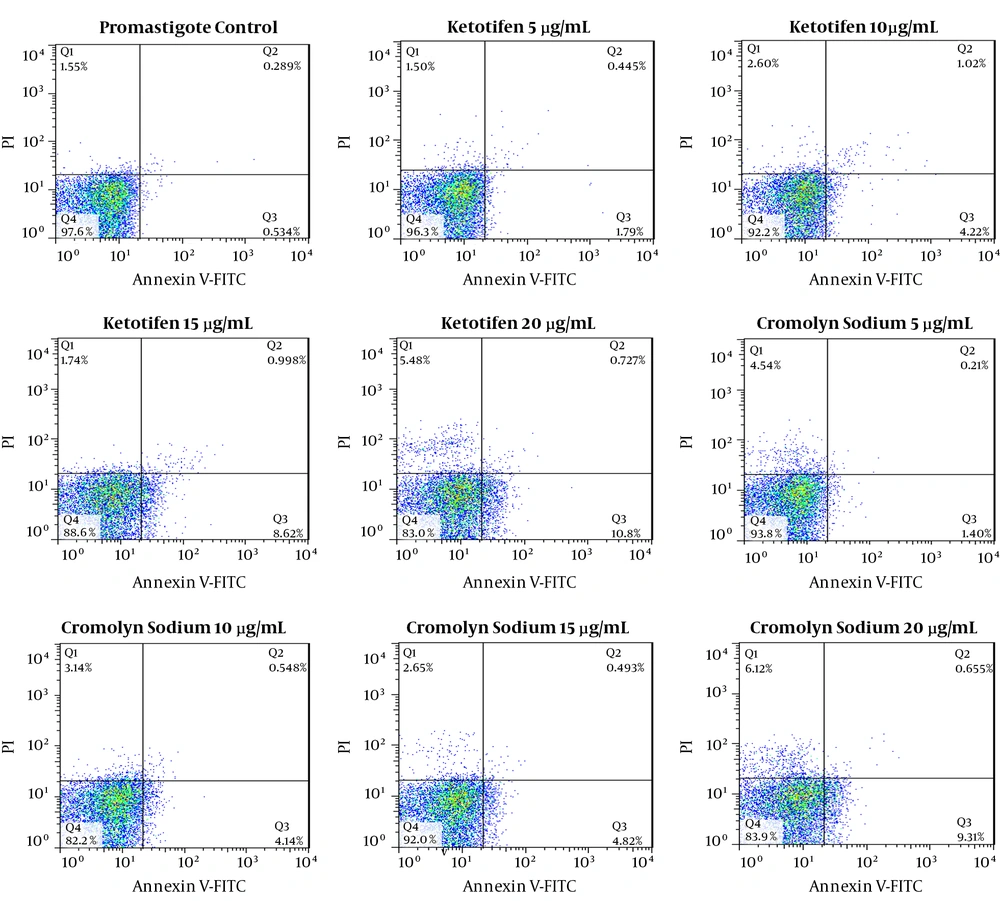
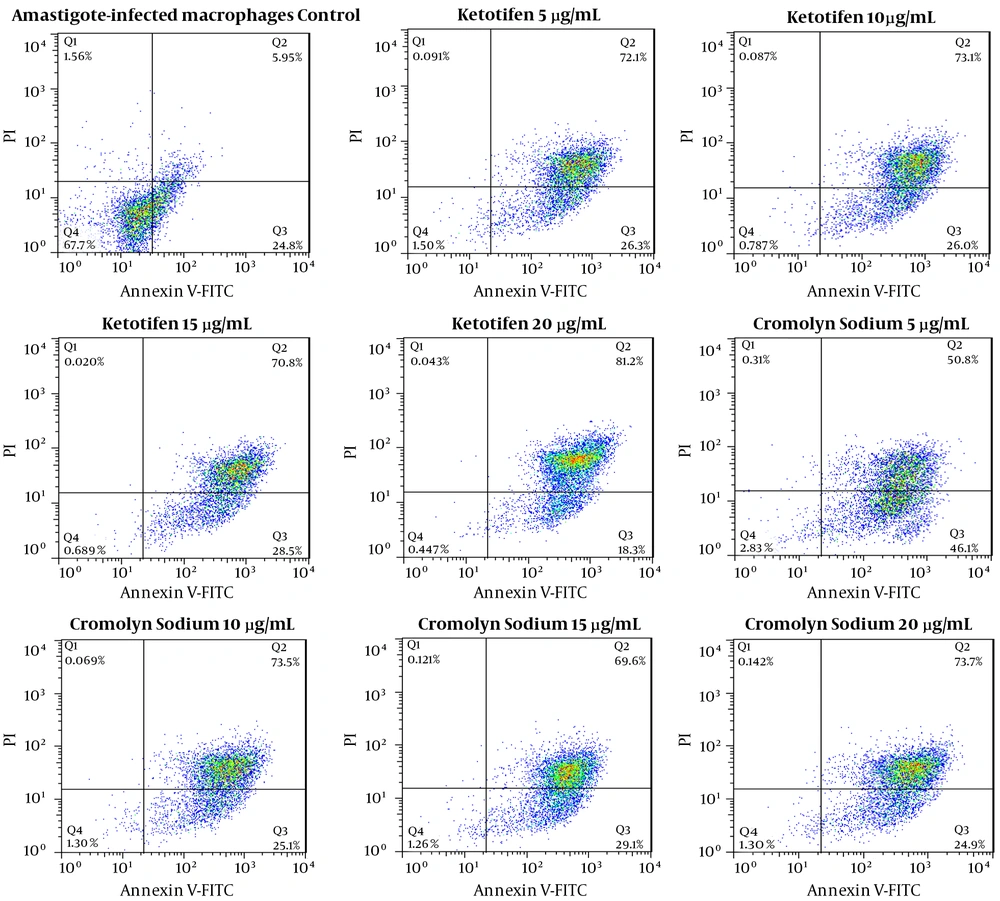
The programmed cell death of Leishmania promastigote cultured in control media, for 72 hours was 0.823%, while that of amastigotes-infected macrophages was 30.75% after 72 hours of culture. The apoptosis rates of promastigotes treated with ketotifen at different concentrations including 5, 10, 15 and 20 µg/mL were 2.23%, 5.24%, 9.61% and 11.52%, respectively while these rates were 98.4%, 99.1%, 99.3%, and 99.5% for amastigotes-infected macrophages, treated with the same doses of the same drug.
In promastigotes treated with 5, 10, 15 and 20 µg/mL of cromolyn sodium, the apoptosis rates were 1.61%, 4.68%, 5.31%, and 9.96%, respectively. The apoptosis rates of amastigotes -infected macrophages were 96.9%, 98.6%, 98.7%, and 98.6% after 72 hours of exposure (Figures 5 and 6). All the results of both promastigotes and amastigotes-infected macrophages were significantly different compared to the controls (P < 0.05).
Flow cytometry analysis of promastigotes following treatment with various concentrations of ketotifen for 72 hours and after labeling with annexin-V and PI. Lower and upper right regions (LR) belong to early and late apoptotic cells (annexin positive) and upper left region (UL) belong to necrotic cells (PI positive)
Flow cytometry analysis of amastigotes, following treatment with various concentrations of cromolyn sodium for 72 hours and after labeling with annexin-V and PI. Lower and upper right regions (LR) belong to early and late apoptotic cells (annexin positive) and upper left region (UL) belong to necrotic cells (PI positive)
5. Discussion
As mentioned previously, drug resistance has been a major obstacle for Leishmania treatment, hence, developing inexpensive, accessible and orally applicable drugs with low side effects is essential, especially when humans are involved as reservoirs. There is an immediate requirement for guides on new inventive drugs (12-14). Hermoso et al. investigated anti-leishmanial effects of Piper elongatum extract against promastigotes. They showed a logical description for the use of this extract (15). Using flow-cytometry, Ghaffarifar et al. studied the effect of artemisinin on L. major growth and their mechanism of action relating to programmed cell death. The authors observed that higher parasite death rates occur when drug doses were increased. The aforementioned study reported that the highest cell death occurred at 100 µg/mL concentration and the lowest cell death occurred at 10 µg/mL (11). Doroodgar et al. showed that tamoxifen-induced apoptosis was dose-dependent as well. Ebrahimisadr et al. reported that artemether had an apoptotic effect on L. major promastigotes and could prevent the growth of both Leishmania in vitro (12, 16). Maroufi et al. reported that cantharidin at different concentrations and times induced apoptosis in L. major promastigotes and macrophages infected with amastigotes (17).
Pinto et al. studied the effect of a series of histamine H1-receptor antagonists on promastigotes and amastigotes of L. infantum. They reported the IC50 value for ketotifen to be 13 µmol, which is comparable to the values obtained by the current study, although the Leishmania strains differed from each other in both studies. This work described, for the first time, the activity of H1-antagonists versus L. infantum and their possible efficiency in a speculative mice model. The evaluation showed that these drugs exert leishmanicidal effects against promastigotes. These researchers postulated that the drugs were the first antihistaminic drugs with anti-leishmanial activities and suggested that the drugs could be used as a platform for drug model studies for visceral leishmaniasis.
The anti-infective activity of H1-antagonist derivatives has also been explained versus Mycobacterium tuberculosis. These drugs have also been described as a calcium channel blocker. Anti-leishmanial and anti-trypanosomal activities of calcium channel blockers have been demonstrated (18). Rezaei et al. studied the effect of ketotifen and cromolyn sodium at different drug doses within 30 to 60 minutes of exposure time on stabilization of cell membrane in order to prevent penetration of Toxoplasma gondii into Balb/c cells. Their results indicated that ketotifen and cromolyn sodium prevent entry of tachyzoites to nucleated cells, both in vitro and in vivo (7).
Ibrahim et al. showed that ketotifen was effective on Plasmodium falciparum both as a stand-alone drug or in combination with malaria common drugs (9). You et al. reported that P. yoelii parasites may be successfully treated with a combination of low dose of ketotifen and chloroquine. This causes the parasites to sometimes die faster with sporadic membranes and vacuoles damages (19). Daryani et al. reported that ketotifen was more effective in preventing penetration of Toxoplasma tachyzoites to macrophages at 10 µg/mL concentration after 60 minutes of exposure. They assumed that T. gondii microtubules also act on each other with dinitroaniline. The cytoskeleton evidently executes significant functions in movement, intrusion, and endodyogeny. Interruption of any of these essential functions might be adopted to kill or disable the parasite.
The researchers have reported that cytochalasin D prevented the entry of T. gondii to peritoneal macrophages and bladder tumor cells following a joint dosage yet did not avert connection. Microfilaments are incriminated to be a frequent place of activity in delaying entry of T. gondii to cells. The researchers have showed that dinitroaniline herbicides avert T. gondii replication. Trifluralin also binds Leishmania tubulin and averts polymerization of subpellicular microtubules. In Plasmodium, Trifluralins restrains fragments of the subpellicular microtubules of gametocytes, also delaying erythrocytic stages and exflagellation of gametocytes (7, 20). Montazeri et al. showed that both ketotifen and pyrimethamine had prophylactic and curative results on acute phases of the disease. The combined treatment with ketotifen and pyrimethamine strongly prevents T. gondii in vivo (21). The used drugs in the current study stabilize cell membrane and prevent parasite entry to the host cells. This research found that drug efficacy was dose and exposure time dependent so that by increasing exposure time and drug concentration, the parasite count decreased.
The MTT assay results showed that upon exposure to high doses of ketotifen and cromolyn sodium, percentage of viable promastigotes decreased when enough time was allowed. Under the said conditions, the viability of promastigotes was significantly different from that of the control group (P < 0.05). The drugs were significantly different from the control in terms of toxic effect (P < 0.05). Drugs used in this study, especially ketotifen, showed lower toxicity yet the same anti-Leishmania effects on both forms as cromolyn sodium did. These drugs were also able to induce apoptosis in promastigotes and amastigotes-infected macrophages of L. major, significantly different from the control groups (P < 0.05). The results showed that drugs used in this study were less effective on induction of programmed cell death in promastigotes, while they exerted greater effect on apoptosis of amastigotes-infected macrophages.
5.1. Conclusions
In this research, because of paucity of information, the apoptotic and leishmanicidal effects of ketotifen and cromolyn sodium were investigated on standard strains of L. major. The primary objective was to investigate the potency of the drugs and report on optimal doses, if proved effective. In contrast to cromolyn sodium, ketotifen showed higher inhibitory effects on both forms of the parasite, and lower toxicities to uninfected macrophages. ketotifen also showed stronger apoptosis induction of programmed cell death on amastigote and promastigote forms of the parasite. Therefore, further in vivo studies are suggested to evaluate the application of different doses of ketotifen for the treatment of leishmaniasis.