1. Background
Staphylococcus epidermidis is an important cause of hospital-acquired opportunistic infection, accounting for more than half of implant-associated bacterial infection (1). Recently, S. epidermidis accounted for 19.3% to 30.1% of many catheter-related blood stream infections (CRBSI), caused by the most common implant catheter (2, 3). Furthermore, S. epidermidis was also estimated to occur in more than 50% of joint-implant infections (4). The strong implant-adhering and biofilm-forming ability of S. epidermidis were blamed for these infections (5).
Biofilms comprise of microorganisms, which are not only bacterial colonies yet also biological systems. It has been documented that biofilms allow S. epidermidis to tolerate high concentrations of antibiotics, protect bacteria from host immune attacks and other environmental stresses, and facilitate spread of bacterial infection (6-8). As a result, treatment of biofilm-associated infections is very challenging and often involves overuse of antibiotics, which may cause the development of resistance. The formation of S. epidermidis biofilms includes adhesion, cell accumulation, and maturation phases (9), therefore, the effects might be different when the antibacterial agents intervene in different phases of biofilms. Clearly, it would be beneficial to identify new agents that can prevent biofilm formation and dissemination.
Tanshinone is the major chemical compound isolated from the root of the Asian medicinal herb, Salvia miltiorrhiza bunge (Danshen) (10, 11). Known as Danshen in China and first recorded as a “top-grade” herb in Sheng Nong’s Herbal Classic (A.D. 102 - 200), S. miltiorrhiza has been used for treating cardiovascular diseases and neurasthenic insomnia for thousands of years in traditional Chinese medicine (12-16). In the recent years, there has been extensive research on chemical constituents in order to determine the effectiveness of application in modern medicine. Tanshinone was proved to exhibit strong antimicrobial activities against a broad range of Gram-positive and Gram-negative bacteria as well as other microorganisms, including viruses (17-19), though its mechanisms of antimicrobial action remain poorly understood. Based on studies of S. epidermidis (18) and S. aureus (19), tanshinone was used to inhibit the formation of S. epidermidis biofilms in this research. However, there has been no report of effects of tanshinone on biofilm formation in any bacteria.
2. Objectives
The researchers’ previously published report on Chinese participants revealed that tanshinone displayed antibacterial and anti-biofilm activities on S. epidermidis (20). Additionally, the aim of this research was to find the most potent inhibitor against S. epidermidis among all tanshinone compounds. The primary goal of this study was to characterize the effects of tanshinone on different phases of biofilm formation in S. epidermidis.
3. Methods
3.1. Bacterial Strains
A biofilm positive S. epidermidis strain SE 1457 was kindly provided by Qu Di, Fudan University, Shanghai, China (21). The strains were maintained in tryptic soy broth media (TSB, Baltimore, MD, USA).
3.2. Growth Curves
Staphylococcus epidermidis biofilms were grown on 96-well plates at 37°C in 200-µL biofilm-inducing medium per well with a starting inoculum of 2 × 106 CFU/mL. During the time points (6, 12, 18, 24, 30, 36, 42, and 48 hours) biofilms were determined by crystal violet staining (crystal violet (CV) assay) and XTT assay (22, 23). For the CV assay, the medium was discarded and the plate was washed three times with PBS, and then stained with 200 µL of 0.5% (w/v) crystal violet, as described previously (24). The bound crystal violet was released from stained cells by adding 75% ethanol and the absorbance of the solution was measured at 590 nm in a microplate reader (KHB, ST-360, Shanghai, CHN). For XTT assay (25), the wells were filled with XTT work liquid (Nanjing KeyGen Biotech. Co., Ltd.) and incubated for an additional two hours in the dark. The absorbance was measured at 450 nm.
3.3. Antimicrobial Agents
Cryptotanshinone, dihydrotanshinone, tanshinone I, and tanshinone IIA were purchased from Chengdu Preferred Biological Technology (Co., Ltd., Chengdu, China), and dissolved in DMSO and filtered through 0.22 µm Millipore filters (Sartorius Co., NY, USA). Vancomycin, ampicillin, oxacillin, cefazolin, and erythromycin were purchased from National Institutes for Food and Drugs Control (Beijing, China) and dissolved in appropriate solvents, according to Clinical and Laboratory Standards Institute (26).
3.4. Minimum Inhibitory Concentrations (MICs)
The MICs of the most abundant constituents of tanshinone and antibiotics, including cryptotanshinone, dihydrotanshinone, tanshinone I, tanshinone IIA, vancomycin, ampicillin, oxacillin, cefazolin, and erythromycin, were determined by the standard micro-titre broth dilution method using 96-well microplates (26). In each well, a 100-µL microbial culture (5 × 105 CFU/mL) was mixed with 100 µL of serially diluted tanshinones or antibiotics (0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 or 128 µg/mL) or solvent, as a control. Microplates were incubated at 37°C for 24 hours. Each sample was tested in triplicates in at least three independent experiments. The MICs were defined as the lowest concentration that yielded no visible bacterial growth after 24 hours.
3.5. Minimum Biofilm Inhibitory Concentrations (MBIC)
Biofilms culture was started as described in growth curves, and the time point of biofilm growth was ascertained according to the growth curves. Staphylococcus epidermidis biofilms were grown on 96-well plates at 37°C in 200 µL biofilm-inducing medium per well with a starting inoculum of 2 × 106 CFU/mL. After culturing for 24 hours, the medium was replaced with 100 µL of serially diluted tanshinones or antibiotics (0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 or 128 µg/mL) or no drug, as the control. Each sample was tested in triplicates in at least three independent experiments. The MBICs were defined as the lowest agent concentration with no visible turbidity after 24 hours (27, 28).
3.6. Effects of Cryptotanshinone on Biofilm Determined by Crystal Violet Staining (CV Assay)
The time points of different phases of biofilm formation were ascertained by the growth curves. The antimicrobial agents and their concentrations were selected according to MICs and MBICs. After culturing for 6, 24 or 48 hours, the medium was replaced with 200 µL of TSB, containing 128 µg/mL cryptotanshinone, 32 µg/mL cryptotanshinone, 32 µg/mL vancomycin or no inhibitor (control). After incubation for an additional 24 hours, the medium was discarded and the plate was washed three times with PBS, and then determined by CV assay, as described previously in growth curves (24).
3.7. Effects of Cryptotanshinone on Biofilm Metabolic Activities (XTT Assay)
The metabolic activity of biofilms formed by S. epidermidis was measured using the tetrazolium salt (XTT) reduction assay as previously described in growth curves. Briefly, after biofilms were pre-grown for 6, 24 or 48 hours, as described above, in the CV assay, the culture medium was changed to 200 µL Mueller-Hinton broth (MHB) in the presence of 128 µg/mL cryptotanshinone, 32 µg/mL cryptotanshinone, 32 µg/mL vancomycin or without an inhibitor (control) (25).
3.8. Effects of Cryptotanshinone on Biofilm Micro Structure Determined by Scanning Electron Microscopy (SEM Assay)
To determine the effects of cryptotanshinone on biofilm microstructure, S. epidermidis biofilms were formed on glass coverslips in six-well plates at 37°C and challenged with cryptotanshinone, vancomycin, or no inhibitor (control). After incubation for 6, 24 or 48 hours, the coverslip was rinsed three times with PBS, followed by gradual dehydration in ethyl-alcohol (30%, 50%, 70%, 70%, 80%, 90% and 100% in v/v). Then, the coverslip was rinsed with tert-Butyl alcohol, and dried in vacuum. After metal spraying, the biofilm was observed by SEM (Inspect F, FEI, USA). Ten views were observed, and the diameter of each view was fixed as 10 µm. The amount of bacteria and morphology was visualized in each view (29, 30).
3.9. Effects of Cryptotanshinone on Expression of Key Genes Involved in Biofilm Formation Determined by Quantitative Reverse-Transcription PCR (qRT-PCR Assay)
It has been reported that icaA, atlE, aap, and luxS are the key genes involved in biofilm formation in S. epidermidis (31). To determine the effects of cryptotanshinone on mRNA expression of these genes during biofilm formation in S. epidermidis, real-time qRT-PCR was performed (32). Briefly, biofilms were grown on six-well plates in the presence of cryptotanshinone or vancomycin or without any inhibitor (control), as described above. Cell pellets were harvested and washed three times with PBS treated with 0.1% diethylpyrocarbonate (DEPC, Sangon, Shanghai, CHN). Total RNA was extracted using the TRIzol® RNA isolation kit (Invitrogen, Madison, WI, USA). The cDNA was synthesized from total RNA using the TransScript II All-in-One First-Strand cDNA Synthesis SuperMix for the qPCR system (TransGen Biotech, Beijing, CHN). Primers specific for S. epidermidis icaA, atlE, aap, and luxS as well as 16S rRNA gene (internal control) were designed using the NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and synthesized by Sangon Biotech (Shanghai, CHN) (Table 1).
| Gene | Primer Sequence (5’ - 3’) | Product Length, bp |
|---|---|---|
| 16SrRNA | 194 | |
| F | CTCGTGTCGTGAGATGTTGG | |
| R | TTTCGCTGCCCTTTGTATTG | |
| icaA | 209 | |
| F | TGGTTGTATCAAGCGAAGTCA | |
| R | CAGGCACTAACATCCAGCATA | |
| atlE | 144 | |
| F | TGGTCTATATTCTATTGCTTGGGG | |
| R | CCTGTTCTGTTATTGACTGTTCCG | |
| aap | 165 | |
| F | AAGCCCCTACAAGAAATGACCTA | |
| R | TGCTTAATAAGGACATAAACGGAGA | |
| luxS | 226 | |
| F | TGACATTCGTTTCAAACAGCCC | |
| R | ATTACAAGCTGGGACTTCGCT |
Primers Used for RT-PCR in This Study
The qRT-PCR was performed using the TransStart Green qPCR SuperMIX (Transgen, Beijing, China) and the qTower® real-time PCR system (Analytik Jena, GER) following the instructions of the manufacturers. The thermo cycling parameters included an initial denaturation at 94°C for 30 seconds, followed by 40 cycles of 94°C for 5 seconds, 53°C for 15 seconds, and 72°C for 10 seconds. Melting-curve analysis was started from initial temperature of 60°C to 95°C, with a gradual increase of 1°C every six second. Specificity of the primers was confirmed by melting curve analysis. The resulting threshold cycle (CT) values were normalized to the CT value of the 16S rRNA gene. Relative expression fold changes were calculated by the 2-ΔΔct formula.
3.10. Statistical Analysis
Data from the CV, XTT, and qRT-PCR assays were expressed as mean values ± standard deviations (SD), and analyzed by the analysis of variance (ANOVA) method, using the SPSS 19.0 software (SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
4. Results
4.1. Susceptibility and Biofilm Formation
The MICs and MBICs are summarized in Table 2. Overall, 2 µg/mL of cryptotanshinone could inhibit S. epidermidis growth and 32 µg/mL of cryptotanshinone could inhibit biofilms. Compared with other compounds from tanshinone, cryptotanshinone showed the strongest effect on S. epidermidis biofilms. The MICs of antibiotics were from 0.125 µg/mL to 2 µg/mL, except erythromycin. However, the MBICs of antibiotics were above 128 µg/mL, except vancomycin. Compared with other antibiotics, only vancomycin could be considered as having an inhibitory effect on S. epidermidis biofilms. Therefore, 32 µg/mL of cryptotanshinone, 32 µg/mL vancomycin and a higher concentration (128 µg/mL) were selected for the following research.
| Antimicrobial Agents | MIC, µg/mL | MBIC, µg/mL |
|---|---|---|
| Tanshinone | ||
| Cryptotanshinone | 2 | 32 |
| Dihydrotanshinone | 8 | 128 |
| Tanshinone I | 8 | 128 |
| Tanshinone IIA | > 128 | > 128 |
| Antibiotics | ||
| Vancomycin | 2 | 32 |
| Ampicillin | 1 | 128 |
| Oxacillin | 1 | > 128 |
| Cefazolin | 0.125 | > 128 |
| Erythromycin | > 128 | > 128 |
Antimicrobial Susceptibility Testing Results
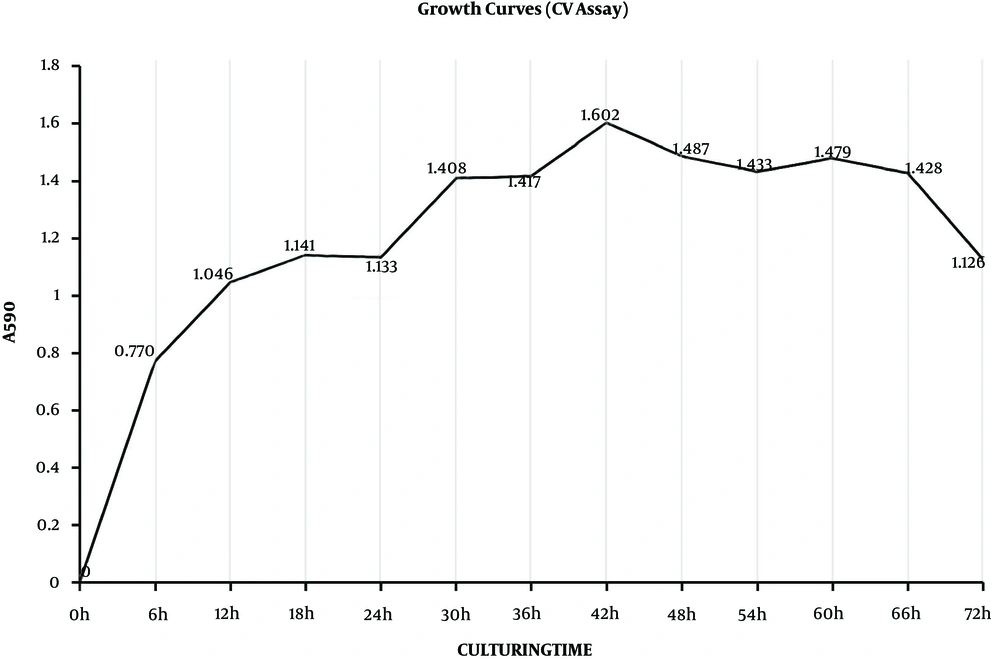
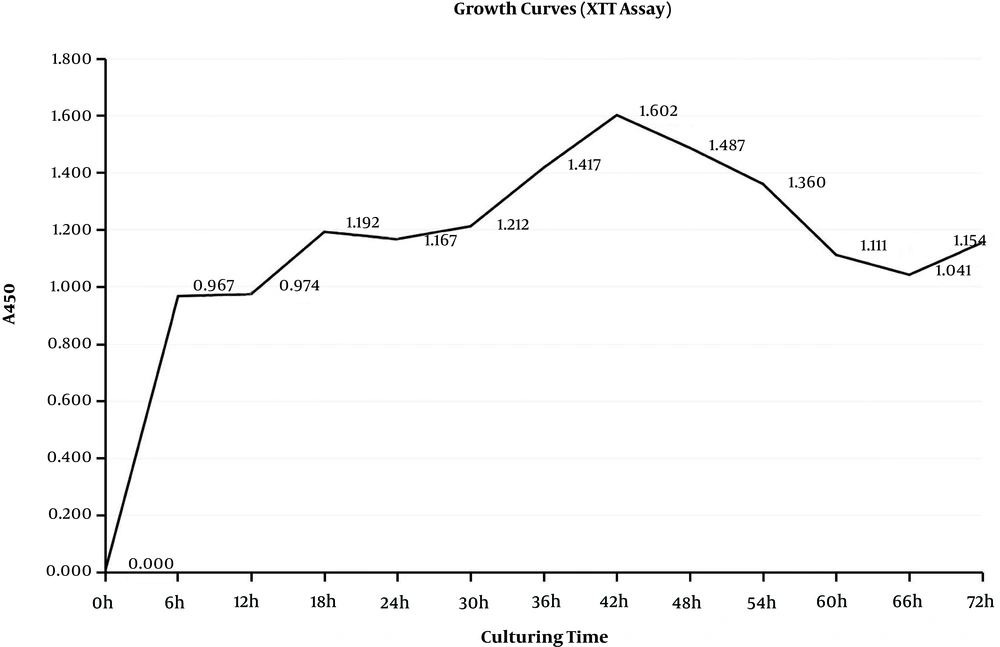
The formation of biofilms is a dynamic process defined as three phases that are adhesion, accumulation, and mature phases. These phases are indicated on the growth curves. The CV assay reflected the matrices of biofilm and the XTT assay reflected the bacteria activities inside biofilms (32). During the adhesion phase, the bacteria adhered to the surface and colonized, which thickened the matrices. From the beginning to 12 hours, matrices of biofilms grew rapidly in the exponential phase and so did bacteria inside biofilms (Figures 1 and 2), which could present the S. epidermidis initial adherence to the surface.
In the accumulation phase, bacteria accumulated with each other and established a three-dimensional, multi-cellular, and multi-layered architecture, in which matrices did not get thick, while the bacteria stayed active. Matrices stayed in the lag phase, which lasted from 12 hours to 24 hours, while metabolic activities stayed in the lag stage from 18 hours to 30 hours. Once the structure frame was built, the biofilms were back growing and became stronger. Therefore, matrices were growing from 24 to 42 hours, and metabolic activities were back growing from 30 hours to 42 hours, which was not as fast as the first stage. Therefore, biofilms were in the accumulation phase from 12 to 42 hours. When biofilms entered the mature phase, bacteria kept a balance inside the biofilm. From 42 to 66 hours, both matrices and metabolic activities grew to the stationary stage. Then, biofilms started to decline because of the nutrient depletion and death of bacteria. Therefore, the time point 6, 24 or 48 hours, respectively, represented the three phases of biofilm formation.
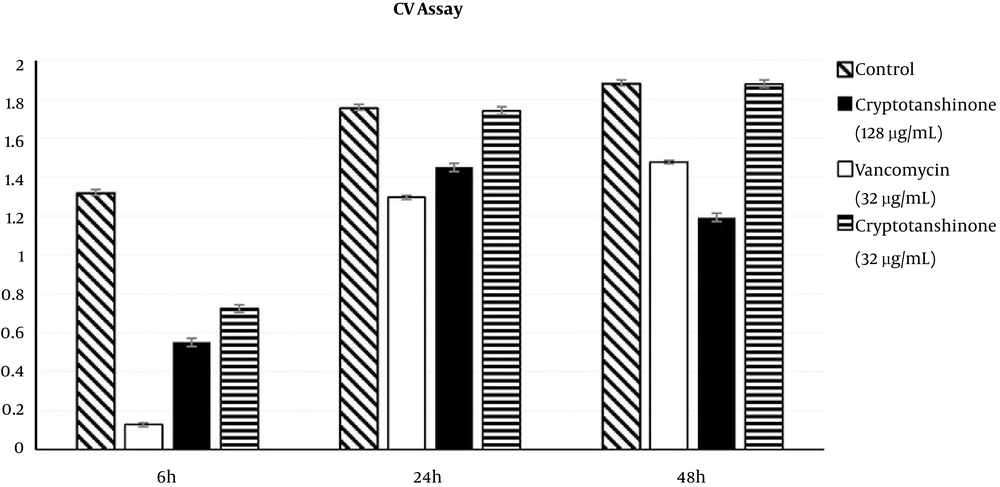
4.2. Effects of Cryptotanshinone on Biofilm Formation Based on the CV Assay
The inhibitory effects on S. epidermidis biofilm formation were determined using two concentrations of cryptotanshinone (32 µg/mL and 128 µg/mL) or vancomycin (32 µg/mL) in the CV assay, in which the optical density was 590 (A590) units and reflected the amount of biofilm matrices formed (Figure 3). In the first phase compared to no treatment control (with A590 of 1.317 ± 0.02), there was a statistically significant decrease in the amount of biofilm matrices formed in the 128 µg/mL cryptotanshinone-treated group (with A590 of 0.55 ± 0.02, P < 0.05) as well as the 32 µg/mL cryptotanshinone-treated group (with A590 of 0.725 ± 0.02, P < 0.05). Compared with the vancomycin-treated group (with A590 of 0.128 ± 0.01), the amount of biofilm matrices formed in the two cryptotanshinone-treated group was much higher (P < 0.05).
In the second phase, the amount of biofilm matrices formed in the 128 µg/mL cryptotanshinone-treated group (with A590 of 1.45 ± 0.0, P < 0.05) significantly decreased compared to the no treatment control (with A590 of 1.753 ± 0.08), and the same was found in the vancomycin-treated group (with A590 of 1.296 ± 0.06, P < 0.05). There was no significant difference in the amount of biofilm matrices formed between the low concentration group (with A590 of 1.439 ± 0.21, P > 0.05) and the control. Compared with the vancomycin-treated group, the amount of biofilm matrices formed in the 128 µg/mL cryptotanshinone group was also higher.
In the third phase, compared to the no treatment control (with A590 of 1.88 ± 0.04), the amount of biofilm matrices formed in the 128 µg/mL cryptotanshinone-treated group (with A590 of 1.193 ± 0.06, P < 0.05) was decreased significantly, and, it was less than the 32 µg/mL vancomycin-treated group (with A590 of 1.478 ± 0.06). However, there was no significant decrease in the 32 µg/mL cryptotanshinone-treated group (with A590 of 1.879 ± 0.05, P < 0.05).
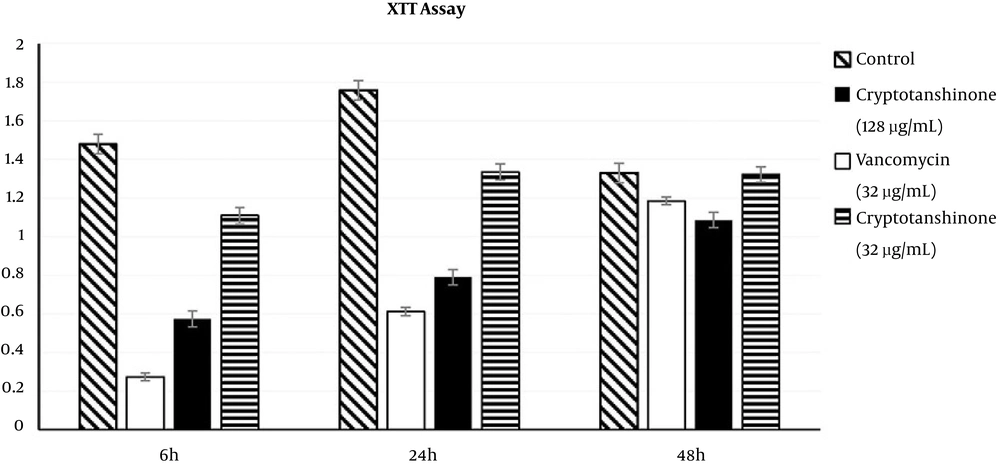
4.3. Effects of Cryptotanshinone on Biofilm Metabolic Activities Based on the XTT Assay
In the XTT assay, metabolic activities were expressed as optical density 450 (A450) (Figure 4). In the first phase, the biofilm metabolic activity was reduced to 61.16% in the 128 µg/mL cryptotanshinone-treated group (A450 = 0.574 ± 0.04, P < 0.05), 24.97% in the 32 µg/mL cryptotanshinone-treated group (A450 =1.109 ± 0.04, P < 0.05) and 81.53% in the vancomycin-treated group (A450 = 0.273 ± 0.02, P < 0.05) compared to the untreated control group (with A450 of 1.478 ± 0.05). There was a significant difference in the biofilm metabolic activity among these three treated groups (P < 0.05). In the second stage, the metabolic activities were 34.81% for vancomycin (A450 = 0.612 ± 0.06), while the activities were 44.94% and 75.94% for cryptotanshinone at the concentration of 128 µg/mL and 32 µg/mL (A450 = 0.79 ± 0.04 and A450 = 1.335 ± 0.06). In the third stage, there was a slight inhibition of cryptotanshinone (128 µg/mL, with A450 = 1.185 ± 0.05) and vancomycin (A450 = 1.085 ± 0.03) (P < 0.05), while there was no inhibitory effect for cryptotanshinone (32 µg/mL, A450 = 1.322 ± 0.04) and only a slight inhibitory effect was observed (P > 0.05).
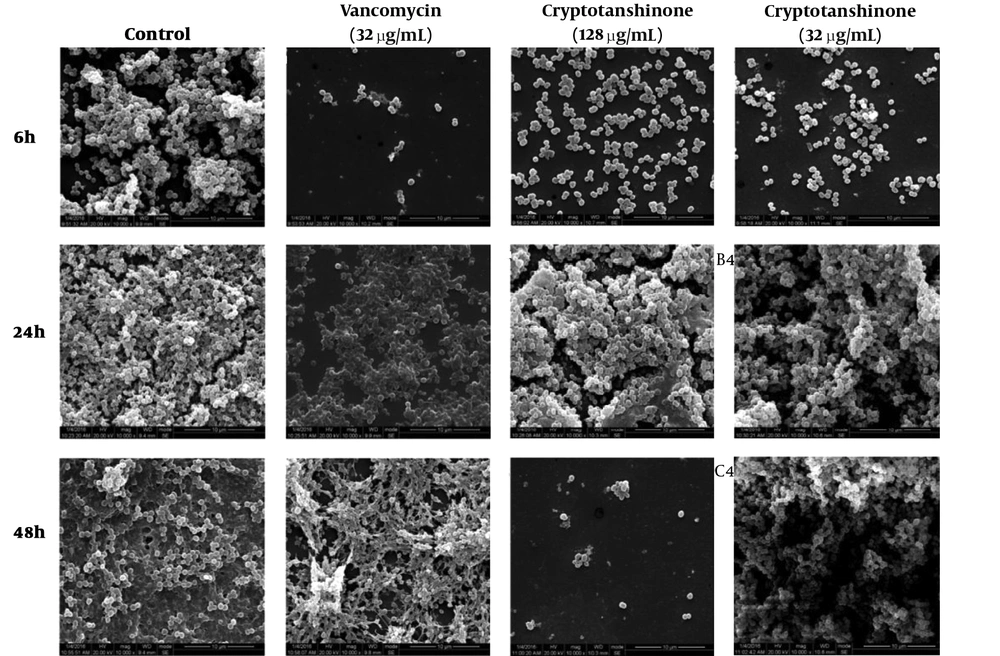
4.4. Effects of Cryptotanshinone on Biofilm Micro Structure Based on SEM
The 10 views equably showed the biofilms, therefore one representative image was picked, as shown in Figure 5. The image of the control sample demonstrates the complex structure of the biofilm filled with cells. In the sight with the same diameter, a significant reduction in bacteria amount was revealed in the image of the cryptotanshinone sample, especially at a higher concentration (128 µg/mL) in the first and third phase, and no reduction in the second phase.
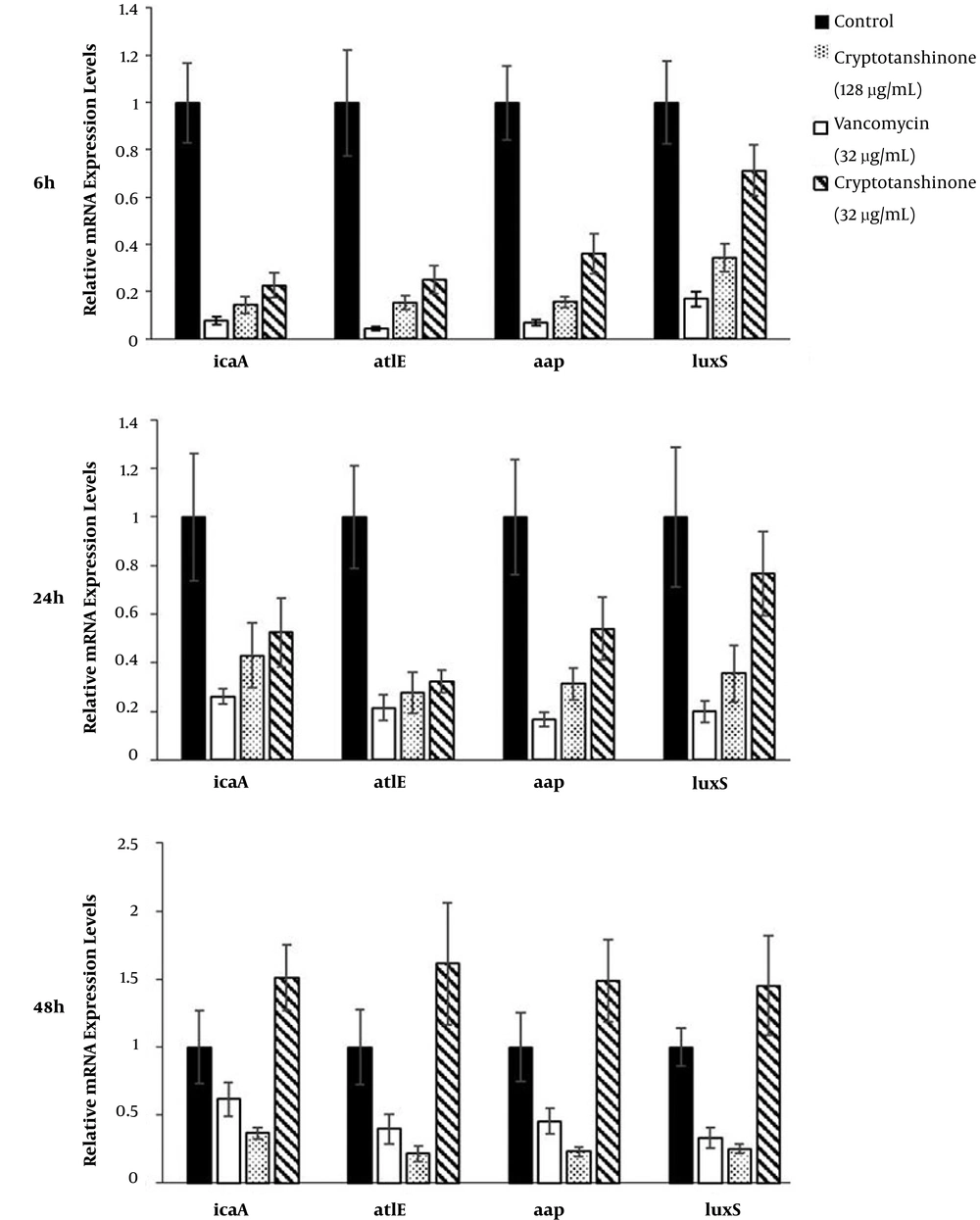
4.5. Effects of Cryptotanshinone on Expression of Key Genes Involved in Biofilm Formation Based on qRT-PCR
The relative expression levels of four key genes (icaA, atlE, aap, and luxS) known to be involved in S. epidermidis biofilm formation were assessed by qRT-PCR, including 16S rRNA gene as an internal control for normalization (Figure 6). The expressions of controls were set as “1”, and the expressions of other groups were correspondingly calculated according to the control. The expressions of ica, atlE, aap, and luxS were down-regulated by cryptotanshinone (128 µg/mL and 32 µg/mL) and vancomycin in the first and second phase (P < 0.05). However, in the third phase, the expressions of the four genes were down-regulated by cryptotanshinone (128 µg/mL) and vancomycin, while up-regulated by cryptotanshinone (32 µg/mL) (P < 0.05).
5. Discussion
Biofilm formation is the main pathogenic factor of S. epidermidis. Biofilm formation was a dynamic process always described in three phases: Initial adhesion (1 to 12 hours), intercellular accumulation (12 to 42 hours), and mature phase (42 to 66 hours). The development of biofilms may allow for aggregation of cell colonies to be increasingly resistant to antibiotics. The antimicrobial agents may show the different extent of inhibitory effect in the different phases of biofilm formation. Therefore, the agents intervened with the three phases of biofilms in this research.
Usually, vancomycin, ampicillin, oxacillin, cefazolin, and erythromycin were common antibiotics used to treat Gram-positive organisms imfrction (26). In this research, planktonic S. epidermidis could be inhibited by vancomycin, ampicillin, oxacillin, and cefazolin, however, S. epidermidis biofilms showed less susceptibility to these antibiotics. Based on MBICs, only vancomycin inhibited S. epidermidis biofilms. At present, vancomycin is commonly used for the treatment of serious infections by Gram-positive bacteria unresponsive to other antibiotics. However, vancomycin has traditionally been considered a nephrotoxic and ototoxic drug, and may increase with drug-resistance. Therefore, vancomycin is always considered as the last resort for the treatment of Gram-positive bacteria. If a new agent that is nontoxic and can inhibit S. epidermidis biofilms as strong as vancomycin was found, it could make great sense.
In the past few years, Danshen and its medical products have become popular around the world because of the broad-spectrum therapeutic effects of this herb and its lack of apparent adverse effects (11, 33). If tanshinone could inhibit the formation of S. epidermidis biofilms, it might be a better choice than vancomycin. Based on inhibitory testing results, the researchers found that cryptotanshinone had the strongest anti-biofilm effect among the extracts from tanshinone. The activities of cryptotanshinone-inhibiting S. epidermidis biofilm in vitro was also examined. The CV assay, XTT assay results, and SEM images showed significant reduction in biofilm matrices, metabolic activities, and morphological changes of S. epidermidis in the intervention of cryptotanshinone as well as vancomycin.
When the cryptotanshinone (128 µg/mL and 32 µg/mL) was added at the initial adhesion stage, the matrices were decreased, the cellular activities inside biofilms were reduced, and the structures of biofilms were destroyed. It was earlier reported that in the adhesion phase, polysaccharide intercellular adhesion (PIA) is made up of sulfated polysaccharides, which allows other bacteria to bind to the already existing biofilms, creating a multilayer biofilm. However, PIA is a major part of the extracellular matrixes synthesized by the gene products of the ica operon (34), which was down-regulated by cryptotanshinone. As a consequence, the cryptotanshinone might decrease the amount of PIA further to decrease the matrices though down-regulating the ica operon.
Along with adhesion, the bacteria produced accumulation-associated rotein (Aap) and autolysin E (AtlE) coded by aap and atlE previously, and contributes to intercellular connections and surface attachment (5, 35). On the whole, PIA forms the major part of the matrixes of biofilms together with ATLE and AAP. When the cryptotanshinone (128 µg/mL) intervened in the adhesion and accumulation phases, the expressions of atlE and aap were decreased, and the polysaccharides and proteins were accordingly reduced, so it was difficult for the bacteria to adhere on the surface or accumulate with each other. Thus, the matrixes of biofilms were fewer, the biofilms were thinner, and same in the metabolic activities of biofilms. Therefore, down-regulation of these genes by cryptotanshinone (128 µg/mL) is consistent with its effects on biofilm foundations.
In mature biofilms, bacteria monitor their own population density or that of other populations by recognizing local concentrations of chemical molecules, according to the QS system, such as the luxS system (36-38). The transcript levels of luxS in mature biofilms treated with cryptotanshinone (128 µg/mL) were also found to be reduced, and accordingly the metabolic activities inside biofilms were decreased. However, when the biofilms grew mature, the cryptotanshinone (32 µg/mL) could not inhibit the growth anymore, which indicated cryptotanshinone had a dose-dependent effect on S. epidermidis biofilms.
5.1. Conclusions
Above all, these results are the first preclinical proofs of evidence for cryptotanshinone capable of inducing S. epidermidis biofilm degradation. Furthermore, cryptotanshinone inhibited three phases of S. epidermidis biofilm, same as vancomycin, due to the down-regulation of biofilm-related genes, such as ica, atlE, aap, and luxS. Therefore, the cryptotanshinone inhibits biofilm formation of S. epidermidis via decreasing PIA, ATLE, and AAP production with a dose-dependent effect. Hence, cryptotanshinone has a broad prospect in treating many kinds of infections not only caused by S. epidermidis biofilms. The researchers propose further in vivo studies on the combination of cryptotanshinone and antibiotics, which will provide new insights into the potential therapeutic effects of cryptotanshinone in the treatment of S. epidermidis biofilm-associated infections.