1. Background
Candida glabrata is an emerging human fungal pathogen, ranking as the second or third common cause of clinical forms of candidosis (1-3), highlighting bloodstream infections due to its unacceptable high mortality rate ranging from 58% to 61% (4). Furthermore, C. glabrata is a species complex comprised of C. glabrata sensu stricto, C. nivariensis, and C. bracarensis (5, 6). On the other hand, the adherence to host tissues, rapid response to changes in the microenvironment, and the ability to secrete hydrolytic enzymes are all considered virulence attributes that enable Candida species to cause disseminated infections in susceptible hosts (7). Particularly, hydrolytic enzymes have been exhaustively studied in C. albicans, yet there is limited information with respect to non-albicans Candida species, such as C. glabrata.
Treatment of C. glabrata infections represents an authentic challenge due to the scarce knowledge of C. glabrata pathogenicity, the increased probability of the organism to develop resistance to azole derivatives, as well as a limited antifungal repertoire (8). The reduced susceptibility of C. glabrata to fluconazole has increased the clinical value of echinocandins, leading ESCMID and IDSA to propose them as the first-line therapy against this yeast (9). However, resistance to echinocandins has also emerged, alarmingly increasing the number of case reports of C. glabrata resistant isolates following echinocandin therapy (10). Thus, local and global antifungal susceptibility surveillance of this threatening pathogen becomes critical in order to document epidemiological shifts and trends. Overall, the aim of this study was to assess the production of lytic enzymes, such as aspartyl proteinase (AP), phospholipase (PP), esterase (ES), hemolysin (HM), DNase (DA), and coagulase (CO) in a subset of C. glabrata sensu stricto clinical isolates, as well as to determine its antifungal susceptibility to echinocandins.
2. Objectives
The present study evaluated the production of some hydrolytic enzymes in a subset of 107 Mexican clinical isolates of C. glabrata sensu stricto. Moreover, its antifungal susceptibility to caspofungin, anidulafungin, and micafungin was determinated.
3. Methods
3.1. Isolates
One hundred and seven isolates of C. glabrata were recollected between 2005 to 2015 at the Department of Microbiology, UANL in Mexico. These isolates were collected from different patient samples and incubated at 37ºC on Sabouraud Dextrose Agar (SDA) (Difco, USA) for 24 hours. Isolates were identified as C. glabrata employing API-20C-AUX strips (bioMérieux, Mexico). In order to confirm the identity of the isolates, DNA extraction by the organic (phenol-chloroform) method was performed (11) and the non-coding ITS region of the rDNA was amplified in a T100 Thermal Cycler (BIO-RAD; Hercules, USA), using the primers IT5 (5’-GGAAGTAAAAGTCGTAACAAGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (12). Once amplicons were purified (Promega; Madison, USA), they were sequenced by Sanger and the obtained sequences were then compared by BLAST with sequence deposits available from the NCBI/GenBank and ISHAM ITS databases. The samples from which the isolates were collected were as follows: 43 (40.2%) from blood, 28 (26.2%) from vaginal swab of Vulvovaginal Candidiasis (VVC) cases, 23 (21.5%) from urine, and 13 (12.1%) from diverse sites, such as peritoneal fluid (8), intra-abdominal abscess (2), cerebrospinal fluid (1), hepatic cyst (1), and bronchial secretion (1). All isolates were preserved on agar slants at -20ºC.
3.2. Enzymatic Activity Assays
The activity of AP, PP, ES, and HM were quantitatively assessed using plate assays with specific test media, such as YCB-BSA medium (Difco & Bio Basic, USA) (13), egg-yolk agar (Difco, USA) (14), tween 80 opacity test medium (Difco & Sigma-Aldrich, USA) (15), and SDA (Difco, USA) with 7% blood (16), respectively. Five microliters of a 107 CFU/mL adjusted suspension was inoculated in specific media, while 10 µL was spotted for the hemolytic assay. Plates were incubated for two, five, seven, and ten days at 37ºC for the determination of HM, PP, AP, and ES, respectively. Enzymatic activities were evaluated using the Pz index and the classification criteria were as follows: very strong, Pz < 0.69; strong, Pz = 0.70 - 0.79; mild, Pz = 0.80 - 0.89; weak, Pz = 0.90 to 0.99; and negative, Pz = 1 (17).
Additionally, DA and CO activities were qualitatively determined employing a medium with methylene green (Difco, USA) and a commercial rabbit plasma (Difco, USA), respectively (18). Both determinations were conducted according to the manufacture’s recommendations.
All assays were done in duplicates. The quality controls used for AP, PP, ES, and HM determinations were C. albicans ATCC 90028 and C. tropicalis ATCC 750, while Staphylococcus aureus ATCC 29213 was utilized for DA and CO assays. Complementarily, in order to compare the results with other published studies, a comprehensive literature review was performed using the platform PubMed of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/pubmed/) under the criteria of search: ‘Candida glabrata hydrolytic enzymes’.
3.3. Antifungal Susceptibility Testing
The antifungal susceptibilities of the isolates to the echinocandins caspofungin, anidulafungin, and micafungin were in accordance with the most recent approved broth microdilution method of CLSI, M27-A4 protocol (19). In brief, two-fold serial dilutions were prepared for each antifungal, and further dilutions were made in RPMI 1640 with MOPS (Hardy Diagnostics, USA). The drug’s working concentrations were 0.015 to 8 µg/mL. Plates were incubated at 35ºC and then read after 24 hours of incubation. Furthermore, C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as quality control organisms. The MIC breakpoints for the interpretation of results were those established by CLSI. Isolates with MICs of ≤ 0.12 µg/mL for anidulafungin and caspofungin and of ≤ 0.06 µg/mL for micafungin were considered susceptible; in counterpart, isolates with MICs of ≥ 0.5 µg/mL for anidulafungin and caspofungin and of ≥ 0.25 µg/mL for micafungin were considered resistant.
3.4. Statistics
The enzymatic profiles of the isolates were correlated based on their clinical origin with chi-square and Fisher’s tests using SPSS version 17.0 for Windows (SPSS; Chicago, USA). P values ≤ 0.05 were considered significant.
4. Results
All analyzed isolates were identified as C. glabrata sensu stricto, according to the sequence analysis of the ITS intergenic region; sequences are available in GenBank (accession numbers: MF187218 to MF187325).
The enzymatic profiles of the C. glabrata sensu stricto isolates evaluated in this study are summarized in Table 1. The AP was secreted by all strains with a very strong activity, excepting for two bloodstream isolates and one strain from vaginal swab. On the other hand, 84 isolates (78.5%) were PP producers with different activity levels, highlighting the finding that all vaginal isolates were very good producers of this enzyme. In counterpart, ES was expressed only by 13 isolates (12.1%), none from urine. Regarding HM, 102 isolates (95%) were hemolytic, exhibiting a very strong activity, especially among isolates recovered from blood, vaginal swab, and diverse other origins. The DA and CO were all negative for the subset of isolates studied. The bloodstream isolates were statistically associated with a very strong activity of AP and HM (P < 0.001), while the vaginal specimens were particularly associated with a very strong production of AP and PP (P < 0.001), as well as HM (P = 0.022). Moreover, the literature review is presented in Table 2, and includes 14 reports; AP and PP were the enzymes more extensively examined.
| Enzyme Evaluated | Origin (N) | |||
|---|---|---|---|---|
| Blood (43) | Urine (23) | Vaginal Swab (28) | Others (13) | |
| Aspartyl proteinase | ||||
| Very strong | 41 | 23 | 27 | 13 |
| Strong | 1 | 0 | 1 | 0 |
| Mild | 1 | 0 | 0 | 0 |
| Weak | 0 | 0 | 0 | 0 |
| Negative | 0 | 0 | 0 | 0 |
| Phospholipase | ||||
| Very strong | 18 | 8 | 27 | 5 |
| Strong | 2 | 11 | 1 | 0 |
| Mild | 3 | 2 | 0 | 5 |
| Weak | 0 | 0 | 0 | 2 |
| Negative | 20 | 2 | 0 | 1 |
| Esterase | ||||
| Very strong | 5 | 0 | 5 | 3 |
| Strong | 0 | 0 | 0 | 0 |
| Mild | 0 | 0 | 0 | 0 |
| Weak | 0 | 0 | 0 | 0 |
| Negative | 38 | 23 | 23 | 10 |
| Hemolysin | ||||
| Very strong | 38 | 11 | 27 | 12 |
| Strong | 2 | 4 | 0 | 0 |
| Mild | 0 | 6 | 0 | 0 |
| Weak | 0 | 2 | 0 | 0 |
| Negative | 3 | 0 | 1 | 1 |
Enzymatic Activities of the Clinical Isolates Studied in This Work
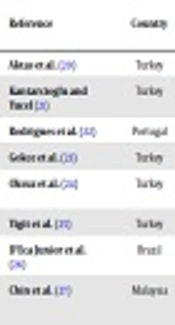
| Reference | Country | No. C. glabrata Isolates (Clinical Origin) | Method (s) of Identification | Enzymatic Activities: No. Isolates with Activity (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| AP | PP | ES | HM | CO | DA | ||||
| Aktas et al. (20) | Turkey | 12 (NS) | API 20C AUX | - | - | 0 (0) | - | - | - |
| Kantarcioglu and Yucel (21) | Turkey | 4 (NS) | Fermentation and assimilation tests | 0 (0) | 1 (25) | - | - | - | - |
| Rodrigues et al. (22) | Portugal | 25 (NS) | API ID 32C | - | - | - | - | 5 (20) | - |
| Gokce et al. (23) | Turkey | 6 (blood) | API ID32C | ND | 0 (0) | - | - | - | - |
| Oksuz et al. (24) | Turkey | 9 (RS = 3, GU = 1, OM = 1, fecal = 1, skin = 3) | API ID 32C | 1 (11.1) | 0 (0) | - | - | - | - |
| Yigit et al. (25) | Turkey | 14 (NS) | API 32C AUX | - | - | - | - | 6 (42.8) | - |
| D'Eca Junior et al. (26) | Brazil | 10 (urine = 7, TS = 3) | Vitek | 6 (60) | 7 (70) | - | - | - | - |
| Chin et al. (27) | Malaysia | 4 (NS) | CHROM agar Candida/ITS sequencing | - | 0 (0) | - | 4 (100) | - | - |
| Tellapragada et al. (28) | India | 13 (VS = 11, blood = 2) | HiChrome Candida agar/Vitek 2/PCR multiplex (ITS) | 2 (15.3) | 0 (0) | 0 (0) | - | - | - |
| Deorukhkar et al. (29) | India | 93 (VS = 41, urine = 24, OM = 11, blood = 8, CSF = 4, miscellaneous = 5) | Assimilation tests/HiChrome Candida agar | 56 (60.2) | 61 (65.5) | - | 62 (66.6) | 45 (48.3) | - |
| Mutlu Sariguzel et al. (30) | Turkey | 2 (blood) | API 20C AUX/ITS sequencing | 0 (0) | 0 (0) | 0 (0) | - | - | - |
| Atalay et al. (31) | Turkey | 14 (blood) | CHROM agar Candida/API 20C AUX | 4 (28.5) | 5 (35.7) | 1 (7.1) | - | - | - |
| Riceto et al. (32) | Brazil | 5 (NS) | ND | 0 (0) | 0 (0) | - | 5 (100) | - | 0 (0) |
| Figueiredo-Carvalho et al. (33) | Brazil | 91 (blood = 25, urine = 13, RS = 10, feces = 9, VS = 7, miscellaneous = 27) | API 20C AUX/CHROM agar Candida/Vitek 2/ITS sequencing | 87 (95.6) | 0 (0) | 51 (56) | 90 (98.9) | - | - |
| This study | Mexico | 107 (blood = 43, VS = 28, urine = 23, miscellaneous = 13) | API 20C AUX/ITS sequencing | 107 (100) | 84 (78.5) | 13 (12.1) | 102 (95.3) | 0 (0) | 0 (0) |
Comprehensive Literature Review
Regarding the antifungal susceptibilities of the isolates, the MIC90 for the three drugs was 0.0625 µg/mL with ranges of 0.0156 to 1 µg/mL for anidulafungin, 0.03125 to 0.5 µg/mL for caspofungin, and 0.03125 to 0.25 µg/mL for micafungin. A very low incidence of echinocandin resistance was detected with only one bloodstream isolate resistant to the three echinocandins, exhibiting a MIC of 1 µg/mL for anidulafungin and caspofungin, and a MIC of 0.5 µg/mL for micafungin.
5. Discussion
The pathogenicity of Candida spp. is determined by the expression of several virulence attributes, such as adherence to host cells, phenotypic switching, the ability to form biofilms, and the capability to produce and secrete hydrolytic enzymes (34, 35). These play a major role in adherence, penetration, invasion, and destruction of host tissue (35), contributing significantly to the pathogenicity of Candida. Some of the most commonly described extracellular hydrolases are AP, lipolytic enzymes, and HM. Importantly, production of these enzymes varies between species and also depends on the source or site of infection (36). In this sense, for example, the current study found an association between C. glabrata sensu stricto isolates from vaginal swabs with a very strong production of AP, PP and H; findings also reported on a strain of C. bracarensis isolated from the same site of infection in a Mexican woman with VVC (18).
Aspartyl proteinase enables Candida invasion and tissue colonization by disruption of host membranes, while controls several steps in innate immune evasion degrading structural and immunological defense proteins (34). In the present study, the researchers found that all strains showed proteolytic activity, with an elevated percentage of isolates with very strong activity of AP (97.2%). As depicted in Table 2, Figueiredo-Carvalho et al. (33) recently reported similar findings in a collection of 91 strains of C. glabrata from diverse clinical sources. However, in the literature, there are several reports with lower variable incidence of proteolytic activity (24, 26, 28, 29, 31) and some authors did not detect such enzymatic activity, probably due to the very limited number of C. glabrata strains they tested (21, 30, 32). On the other hand, PP and ES are extracellular lipolytic enzymes that contributed to the virulence of Candida spp., possibly through damage to the host cell membrane by digestion of lipids, facilitating tissue invasion as well as nutrient acquisition (35)
In this work, the researchers detected 84 isolates (78.5%) with PP activity at different production levels, with these observations agreeing closely with certain studies (26, 29) and more distantly with some additional reports (21, 31); as opposed, other authors did not find PP activity (23, 24, 27, 28, 30, 32), even in collections of numerous strains of C. glabrata (33). Otherwise, as expected, a limited number of isolates (12.1%) exhibited ES activity, agreeing with some studies (20, 28, 30, 31) and in contrast to the work of Figueiredo-Carvalho et al. (33), who reported an incidence of 56%, and further suggested that the production of this enzyme may vary according to the clinical source or the geographic region, from which the strains were isolated.
Hemolysin is important for elemental iron uptake from hemoglobin through lysis of red blood cells. Thus, this putative virulence factor enables pathogen survival and persistence in the host (37). In the present study only five isolates were unable to produce HM, these findings are in agreement with previous publications (27, 32, 33), reflecting the importance of this key virulence attribute for C. glabrata. In fact, the hemolytic activity has been demonstrated as necessary for virulence in this yeast (38). On the other hand, although less studied, other extracellular lytic enzymes that also contribute to the pathogenic fitness of Candida spp. are CO and DA. In this study, these enzymes were not produced among the studied isolates, in contrast with scarce works (22, 25, 29) and in agreement with the findings communicated by Riceto et al. (32).
Echinocandins are the front-line antifungals for the treatment of candidemia and other forms of invasive candidiasis due to C. glabrata (9). Although case reports were initially infrequent, echinocandin resistance in Candida spp. is emerging, particularly in C. glabrata. This phenomenon mostly occurs in patients with long periods of echinocandin treatment or prophylaxis, and it is principally related to mutations in hot-spot regions of FKS gene, which participate in the production of 1,3-β-D-glucan synthase (39), manifesting phenotypically with magnitudes of change in the MIC values.
In the present study, echinocandins exhibited excellent in vitro activity against the studied isolates, with only one strain (0.9%) resistant to the three candins tested. Similar results were previously reported by Morales-Lopez et al. (40) in an Argentinian collection of 114 clinical strains of C. glabrata sensu stricto. Even though the incidence of echinocandin-resistant isolates of C. glabrata is low at the moment, it seems to be on the rise, and the available information is fragmentary, coming from single case reports or series with limited number of patients. The conduction of surveillance studies such as the current research is essential in order to monitor antifungal resistance and to announce the regional epidemiology of C. glabrata sensu stricto.
5.1. Conclusions
The bloodstream isolates of C. glabrata sensu stricto were associated with a very strong activity of AP and HM, while those recovered from vaginal swabs were particularly associated with a very strong production of AP, PP and HM. On the other hand, echinocandin resistance was rare in the subset of isolates evaluated.