1. Background
Malaria is the most important vector-borne infectious and parasitic disease, which is transmitted through mosquito bites. Malaria transmission occurs in many parts of the world, threatening more than 40% of the world’s population. Indeed, about 97 countries in the world are considered as malaria-endemic areas. According to the World Health Organization (WHO), about 216 million malaria cases occurred worldwide in 2016, causing 445,000 deaths (1). Iran is considered one of the malaria-endemic countries of the Middle East region. Malaria is one of the most important health problems in the southern and southeastern parts of the country, including Sistan and Baluchistan, Hormozgan, and Kerman provinces. The dominant species of malaria in Iran is Plasmodium vivax, while P. falciparum has been reported in 10 - 15% of the cases (2, 3). Anopheles stephensi and An. culicifacies are the main vectors of malaria in Iran. Furthermore, An. superpictus, An. sacharovi, An. dthali, An. pulcherrimus, and An. fluviatilis species complex is responsible for malaria transmission in Iran (4, 5).
The impact of malaria on public health and health promotion elements, as well as its social and economic effects on communities’ development, has caused international organizations to focus on this disease, ultimately codifying a comprehensive plan for control, elimination, and eradication of malaria (6, 7). In recent years, the number of malaria cases has decreased significantly in Iran (11,460 to 705 cases during 2008 to 2016) (1). Because of the successful implementation of malaria prevention and control programs in Iran and the subsequent reduction of malaria cases in recent years, the malaria elimination program has been launched in the country with technical support from the WHO since 2009 (2, 7, 8). The main goal of the malaria elimination plan is to stop malaria transmission locally. To achieve the goal of malaria elimination, all positive cases should be promptly diagnosed and treated, especially asymptomatic and low parasitic ones that cannot be detected by routine procedures (9).
Following the development of novel and sensitive methods for malaria detection, especially molecular techniques, asymptomatic malaria cases are now detectable (10). Nonetheless, treated patients might act as an asymptomatic reservoir in the establishment of the malaria transmission cycle (11). Many studies have shown that asymptomatic carriers, as unknown reservoirs, play important roles in establishing the malaria transmission cycle in different parts of the world (12). Because of the importance of this issue in the malaria elimination program, it attracted the attention of several researchers and led to many reports about the presence of asymptomatic cases in Africa, South America, and Asia (12), including Nigeria (13), Zambia (14), Gabon (15), Senegal (16), Brazil (17), Colombia (18), Yemen (19), Thailand (20), Cambodia (21), Solomon Islands (10), and Minab district of Iran (2). On the contrary, some studies have shown no cases of asymptomatic malaria cases in Sri Lanka and Iran (3, 22-24).
2. Objectives
The present study aimed to follow up and monitor malaria treated cases using sensitive molecular tools (nested-polymerase chain reaction (PCR)), as well as microscopic and rapid diagnostic test (RDT) techniques in Bashagard district, Hormozgan province, Iran. The results of this study would facilitate the implementation of malaria elimination programs in Hormozgan province and the entire country.
3. Methods
3.1. Study Area
Hormozgan province is located in the southern part of Iran and is considered an endemic area for malaria, accounting for 11.5% (81 of 705 cases) of all Iranian malaria cases in 2016 (Figure 1). This cross-sectional survey was conducted in Bashagard district of Hormozgan province [(26 ° 21’58 ”N 57 ° 35’48 ”E); area = 9,200 km2] during 12 months from 2015 to 2016. According to the 2017 census, the population of Bashagard district was 35931 in 8615 families. Its average annual relative humidity is 46.2% and the average annual precipitation is about 150 mm. In addition, the daily temperature varies from 7.7°C to 44.2°C in this district (www.weather.ir). These features have created a good condition for malaria transmission in this region throughout the year. Currently, the malaria elimination program is ongoing in Bashagard district (API < 1/1000) (23).
3.2. Sampling Methods
Five hundred people were randomly selected from the entire population of Bashagard district. After the initial review, 208 out of 500 were found as the treated cases of malaria. All 208 cases (125 women and 83 men) were included in the study. The cases had been treated according to the National Malaria Treatment Guideline (I.R. Iran, 3rd edition). The selected cases were evaluated for Plasmodium infection rate by using microscopic, RDT, and nested-PCR (using 18ssrRNA) techniques. The inclusion criteria of the study were having a history of malaria treatment and not having the symptoms and clinical signs of malaria. Individuals with a history of travel to other malaria-endemic areas in the past year were excluded from the study. Before starting the project, all participants signed consent forms and agreed to complete the trial. After the interviews and registration of the profiles, 3 mL of blood samples were taken from each participant for the diagnosis of the malaria parasite.
3.3. Microscopy
The microscopic method is the gold standard for the detection of malaria (25-28). Briefly, after scrubbing the finger with 70% ethanol, a small scratch was created by a lancet. Then, thick and thin blood smears were prepared from each person according to the standard method. All the slides were stained with Giemsa method. After drying, the thin smear was fixed with methanol; the slides were stained with Giemsa 10% for 20 minutes and examined at 1000x immersion oil for the diagnosis of malaria parasite by expert microscopists (29). The RDT was done in the field. After transferring samples to the laboratory, nested PCR and microscopic test were done by an experienced person blind to the results of the RDT. In order to conduct the follow-up, 30 and 60 days after the initial sampling, new blood smears were taken and examined for Plasmodium using the microscopic test.
3.4. Rapid Diagnostic Test
The rapid diagnostic test is an immuno-chromatographic experiment, which recognizes the presence of a specific malaria antigen in the blood sample. The kit has a two-line strip previously coated with two monoclonal antibodies, one against the pan-specific lactate dehydrogenase (pLDH) of all Plasmodium species and the other against P. falciparum histidine-rich protein 2 (HRP2). In this study, 5 µL of each patient’s blood sample was added to the sample well in the test card. Then, 60 µL of the buffer solution was added to the well. After about 15 minutes, the formation of specific bands showed if the patient was infected with human malaria species (29). In so doing, the Combo test kit was used (Premier Medical Corporation Ltd., Mumbai, India) and the test was performed according to the manufacturer’s instructions.
3.5. Nested-PCR
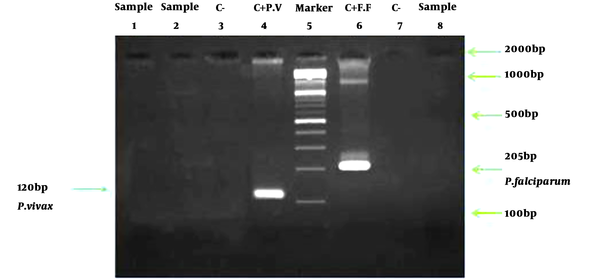
In this study, the nested-PCR method was used to diagnose Plasmodium species. This method benefits from high sensitivity and accuracy in detecting asymptomatic cases (30). In this study, the Promega kit was used to extract DNA from blood samples (Promega, Madison, WI, USA). Additionally, the molecular diagnostic method was carried out based on research by Snounou et al. (31). Positive and negative controls were tested in each series of reactions. Accordingly, a sample was considered positive for P. vivax and P. falciparum if a 120- and 205-base-pair fragment was identified, respectively (2, 25).
4. Results
To investigate the presence of asymptomatic malaria reservoirs in Bashagard district, 208 malaria treated cases with no specific malaria symptoms were included in this study. The demographic characteristics of the participants are shown in Table 1. Of the total number of 208 participants in the study, 39.9% were male and 61.1% were male. None of these participants had symptoms of malaria before sampling. Then, they were assessed using microscopic, RDT, and nested-PCR methods, the results of which are given as follows. All of them had a history of P. vivax and their treatments were based on the national malaria treatment protocol. The results of all microscopic slides were negative. In order to increase the accuracy of the results, all microscopic slides were re-examined by an expert microscopist. The RDT was used as a complementary method for all 208 samples. The results of all samples were negative using the RDT kits. Despite the use of a sensitive molecular method for all specimens, no positive cases were found in the nested-PCR method (Figure 2).
| Parameter | No. (%) |
|---|---|
| Sex | |
| Female | 125 (61.1) |
| Male | 83 (39.9) |
| Total | 208 (100) |
| Age group | |
| 5 - 20 | 83 (39.9) |
| 21 - 35 | 46 (22.12) |
| 36 - 50 | 57 (27.4) |
| 51 - 65 | 22 (10.58) |
| Total | 208 (100) |
| Malaria history | |
| Yes | 208 (100) |
| No | 0 |
| Total | 208 (100) |
Distribution of the Sample Based on Age Groups, Sex, and History of Malaria Infection
5. Discussion
Malaria elimination is the common goal of WHO and Iran’s health system. This study was conducted in accordance with the malaria elimination program in Iran. Despite the use of a sensitive molecular technique, microscopic methods, and RDT, there were no cases of asymptomatic malaria among treated cases in Bashagard district. In recent years, local malaria transmission has decreased in Iran and according to the WHO classification, Iran is a candidate for the malaria elimination phase (API < 1/1000) (7). In the elimination phase, all effective factors in creating a malaria transmission cycle in the region should be considered. Besides, all positive cases must be diagnosed and timely treated, especially low parasite and asymptomatic cases that are not detectable by routine methods. Moreover, malaria treated cases can act as asymptomatic reservoirs in malaria-endemic areas and consequently, they should be taken into consideration (11). In the malaria elimination program, in addition to microscopic and RDT methods, more robust and sensitive diagnostic techniques, including tools to enable the detection of parasite carriers (low parasitism, asymptomatic infections) are required. In this study, microscopic, serology, and molecular methods were used simultaneously to increase sensitivity in the diagnosis of Plasmodium species.
The findings showed that because of the great surveillance system for case finding, diagnosis, and treatment of malaria cases, there were no positive malaria treated cases in Bashagard district. It can be concluded that the timely and appropriate treatment of malaria cases, as well as monitoring of the treatment process, is one of the key strategies for the control and elimination of malaria. These results supported those of the previous investigations in Bashagard district, reporting no cases of asymptomatic malaria (3, 23, 24). The results were also in agreement with those of a study performed by Amirshekari et al. in Kerman province, which revealed no asymptomatic malaria cases among indigenous people (26). In contrast, the study by Shahbazi et al. showed two asymptomatic carriers of malaria in treated cases in Bashagard and Minab districts (11). Another survey in Minab district also indicated some cases of asymptomatic malaria. The difference between the study results might be attributed to differences in the monitoring of health systems, as well as to specific climatic and environmental conditions (2).
Several reports have demonstrated the high prevalence of asymptomatic malaria in the world, especially in Central and South America, Africa, and East Asia, which is not aligned with the present study results. This contradiction might have resulted from genetic diversity in humans, parasites, and carriers of malaria, different weather conditions, demographic characteristics, living conditions, population movements, and differences in surveillance systems (27, 28). The strengths of this study included its high sensitivity and accuracy of diagnosis resulted from the proper sample size and using a sensitive molecular method along with microscopic and RDT methods. However, the study limitations were related to field sampling and following up of cases due to the displacement of the study population.
5.1. Conclusions
It can be claimed that the robust malaria surveillance system (detection, diagnosis, and treatment of positive cases of malaria and monitoring of the treatment process) has caused the malaria elimination program to be successfully implemented in Bashagard district. According to the malaria elimination program, a series of research is necessary for the study region to monitor and evaluate asymptomatic malaria in high-risk areas.