1. Background
Antimicrobial resistance is a growing healthcare concern worldwide. Gram-negative, non-fermenter, rod-shaped bacteria including Acinetobacter spp. and Pseudomonas spp. are significant causes of nosocomial infections. These bacteria are a serious challenge to health care systems because they are optimally successful pathogens to develop antimicrobial resistance. Acinetobacter spp. are opportunistic organisms that can cause several infections ranging from superficial skin and soft-tissue infections to more severe diseases such as pneumonia and bloodstream infection (BSI) (1-3). The results of a cohort study in the United States showed that the mortality rate of bloodstream infections caused by Acinetobacter spp. was 49.6% (4). Pseudomonas spp., especially Pseudomonas aeruginosa, as renowned opportunistic bacteria, was the sixth cause of healthcare-associated infections and the third most common Gram-negative bacterium causing BSI among 11,282 patients in 183 hospitals of the United States (5).
The report from India revealed that the overall prevalence rates of Acinetobacter and Pseudomonas spp. were respectively 1048 (5.6%) and 828 (4.4%) among 18,695 isolates from blood samples between 2008 and 2014 (6). In a multicenter study in Iran by Poorabbas et al. among all 858 isolates obtained from positive sterile body fluid cultures, 95 (11.07%) and 67 (7.80%) of them were Pseudomonas and Acinetobacter spp., respectively (7). Currently, these pathogens have turned out to be a “red-alert” because of their rapid emergence of resistance following the overuse and misuse of antibiotics and the increased incidence and the worldwide spread of multidrug-resistant (MDR) isolates (8).
Antimicrobial resistance surveillance is crucial for timely administration of the proper empirical antibiotics to reduce the unfavorable complications, the length of hospital stay, mortality, and health care costs (9). Antimicrobial resistance surveillance should be carried out and carefully observed in each area. Unfortunately, there are no sufficient data from developing countries on the trend of antimicrobial resistance of such microorganisms causing BSIs.
2. Objectives
In this study, we aimed to report the seven-year trend of antimicrobial resistance of Acinetobacter and Pseudomonas spp. causing BSIs in Shiraz, southern Iran, during 2010 - 2016.
3. Methods
3.1. Design, Period and Location of the Study
This retrospective descriptive study was conducted at Professor Alborzi Clinical Microbiology Research Center (PACMRC), a referral microbiology laboratory in Shiraz, southern Iran, on the recorded blood cultures from 2010 to 2016. We investigated the antimicrobial resistance of all Acinetobacter and Pseudomonas spp. isolated from blood specimens submitted for culture in an automated blood culture system (BACTEC® BD).
The blood samples were sent from Nemazee Teaching Hospital and four other teaching hospitals (Shahid Dastghaib, Rajaiee, Chamran, and Zeinabieh hospitals) affiliated to Shiraz University of Medical Sciences, and eight other nonteaching hospitals (Amir, Dena, Markazi, Ghadir, Kowsar, Ghotbedin, Ordibehesht, and Moslemin hospitals) in Shiraz. The majority of the samples were from Nemazee teaching hospital, a tertiary hospital with more than 1000 beds including surgical and medical wards, emergency room, and intensive care units.
3.2. Identification and Confirmation of the Isolated Bacteria
The identification and confirmation of the isolated bacteria were performed using biochemical methods (7). The biochemical characterization was done by performing oxidase reaction, pigmentation or mucoidity, growth at 42°C, growth on MacConkey agar, and using two commercially available miniaturized multi-test identification systems, i.e., API (bio Merieux SA, Marcy-1, Etoile, France) and Microgen (Microbiology International, UK).
3.3. Antimicrobial Susceptibility Testing
The disk diffusion method was used to assess susceptibility to 19 antimicrobial agents including amikacin (30 μg), ampicillin-sulbactam (10/10 μg), aztreonam (30 μg), cefepime (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), trimethoprim and sulfamethoxazole (1.25/23.75 μg), colistin (10 μg), gentamicin (10 μg), levofloxacin (5 μg), Imipenem (10 μg), meropenem (10 μg), piperacillin-tazobactam (100/10 μg), tetracycline (30 μg), ticarcillin (75 μg), tigecycline (30 μg), and tobramycin (10 μg).
All the isolates were tested by using cation-adjusted Mueller-Hinton agar (Merck Co., Germany). The results were interpreted according to the clinical and laboratory standards institute (CLSI) guideline (10). To determine the resistance of Acinetobacter spp. to colistin, we used the disk diffusion test based on provisional zone diameter breakpoints suggested by Galani et al. (11). We considered inhibition zone diameter breakpoints of ≥ 14 mm as susceptible. Extended-spectrum beta-lactamases (ESBL) production was determined according to the CLSI guideline using cefotaxime, cefotaxime-clavulanic acid (30/10 μg), ceftazidime, and ceftazidime–clavulanic acid disks (30/10 μg) (10).
3.4. Statistical Analysis
We merged the intermediate resistant pathogens into resistant ones to report the susceptibility rate, on the ground that the clinical approach to both is the same. For the purpose of analysis, data were grouped into three episodes: 2010 to 2011, 2012 to 2013, and 2014 to 2016. The statistical analyses were done by SPSS version 16 software. The chi-square and Fisher’s exact tests were used to determine the significance of antimicrobial resistance trends over the three periods of study. The P values of ≤ 0.05 were considered statistically significant.
4. Results
The number and percentage of microorganisms in each period are shown in Table 1. In the first episode (2010 - 2012), Acinetobacter spp. and Pseudomonas spp. were ranked as the fourth and fifth common bacteria causing BSIs. In 2012 - 2013, Pseudomonas spp. and Acinetobacter spp. were the third and fourth prevalent isolated bacteria, respectively. In the last episode, Pseudomonas spp. was the most frequent bacteria and Acinetobacter spp. was ranked third.
| Bacteria | 2010 to 2011 | 2012 to 2013 | 2014 to 2016 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Acinetobacter spp. | 91 | 6.64 | 180 | 11.05 | 177 | 11.71 | 448 | 9.93 |
| Brucella spp. | 14 | 1.02 | 18 | 1.10 | 5 | 0.33 | 37 | 0.82 |
| Citrobacter spp. | 18 | 1.31 | 5 | 0.31 | 5 | 0.33 | 28 | 0.62 |
| Escherichia coli | 249 | 18.16 | 225 | 13.81 | 146 | 9.66 | 620 | 13.74 |
| Enterobacter spp. | 66 | 4.81 | 47 | 2.89 | 59 | 3.90 | 172 | 3.81 |
| Enterococcus spp. | 122 | 8.90 | 175 | 10.74 | 181 | 11.97 | 478 | 10.59 |
| Klebsiella spp. | 72 | 5.25 | 109 | 6.69 | 100 | 6.61 | 281 | 6.23 |
| Pseudomonas spp. | 90 | 6.56 | 194 | 11.91 | 198 | 13.10 | 482 | 10.68 |
| Salmonella spp. | 14 | 1.02 | 9 | 0.55 | 17 | 1.12 | 40 | 0.89 |
| Serratia spp. | 65 | 4.74 | 36 | 2.21 | 30 | 1.98 | 131 | 2.90 |
| Staphylococcus aureus | 283 | 20.64 | 351 | 21.55 | 150 | 9.92 | 784 | 17.38 |
| Stenotrophomonas maltophilia | 39 | 2.84 | 34 | 2.09 | 175 | 11.57 | 248 | 5.50 |
| Streptococcus pneumoniae | 27 | 1.97 | 22 | 1.35 | 12 | 0.79 | 61 | 1.35 |
| Streptococcus spp. | 86 | 6.27 | 93 | 5.71 | 121 | 8.00 | 300 | 6.65 |
| Others | 135 | 9.85 | 131 | 8.04 | 136 | 8.99 | 402 | 8.91 |
| Totals | 1371 | 100 | 1629 | 100 | 1512 | 100 | 4512 | 100 |
a Methicillin-resistant coagulase-negative staphylococci (MRCoNS) is a remarkable sample contaminant; therefore, the results are reported in this table excluding these bacteria.
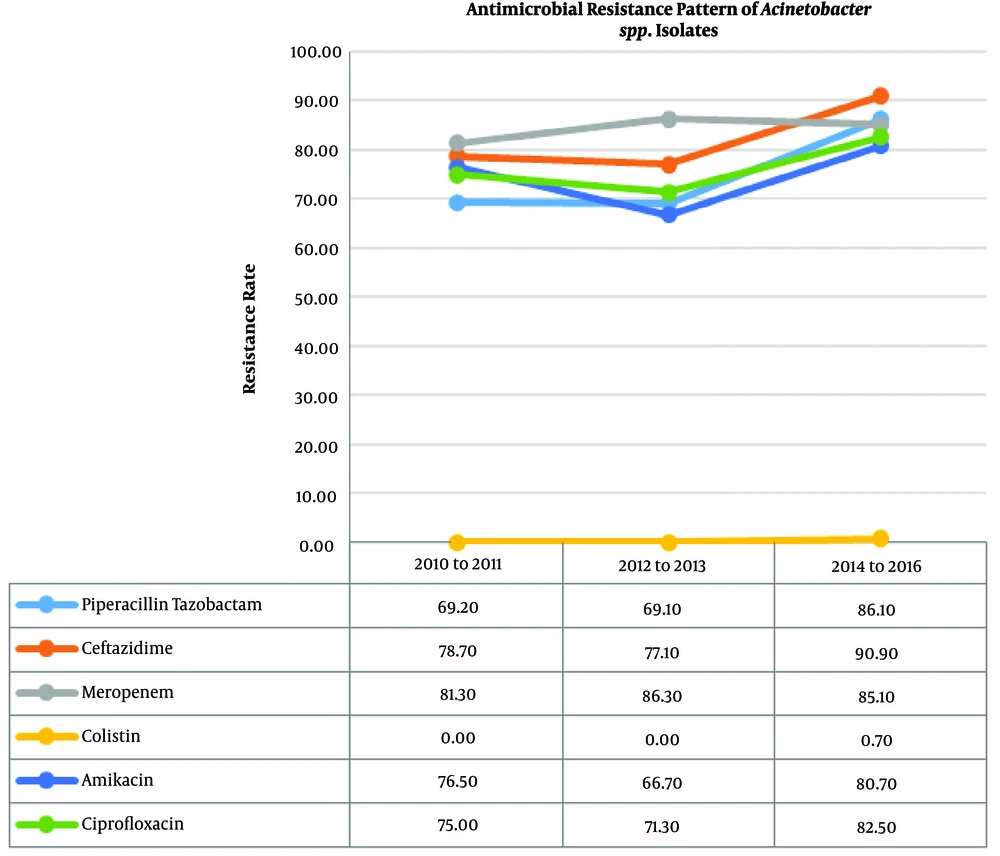
The total number of isolated Acinetobacter spp. was 439. There was a statistically significant increase in the antibacterial resistance trend of Acinetobacter spp. against amikacin (P = 0.011), cefepime (P = 0.011), cefotaxime (P = 0.029), ceftazidime (P = 0.001), ciprofloxacin (P = 0.048), and piperacillin-tazobactam (P < 0.001). The pattern of resistance to the frequently used anti-Acinetobacter antibiotics is shown in Table 2 and Figure 1. The resistance of Acinetobacter spp. to colistin increased from zero percent in both 2010 - 2011 and 2012 - 2013 periods to 0.7% in 2014 - 2016.
| Antibiotics | Susceptibility | P Value | |||||
|---|---|---|---|---|---|---|---|
| 2010 to 2011 (N = 90) | 2012 to 2013 (N = 194) | 2014 to 2016 (N = 189) | |||||
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | ||
| Penicillins | |||||||
| Piperacillin | ND | ND | 27 (20.5) | 105 (79.5) | 7 (12.5) | 49 (87.5) | 0.220 |
| Ticarcillin | 12 (14.6) | 70 (85.4) | 31 (18.1) | 140 (81.9) | 13 (16.3) | 67 (83.8) | 0.792 |
| Beta-lactam/beta-lactamase inhibitors combinations | |||||||
| Ampicillin-sulbactam | ND | ND | ND | ND | 27 (17.5) | 127 (82.5) | - |
| Piperacillin-tazobactam | 24 (30.8) | 54 (69.2) | 54 (30.9) | 121 (69.1) | 23 (13.9) | 142 (86.1) | < 0.001 |
| Cephalosporins | |||||||
| Cefepime | 9 (10.5) | 77 (89.5) | 37 (22.6) | 127 (77.4) | 19 (11.7) | 144 (88.3) | 0.011 |
| Cefotaxime | ND | ND | 20 (11.8) | 150 (88.2) | 8 (4.8) | 157 (95.2) | 0.029 |
| Ceftazidime | 19 (21.3) | 70 (78.7) | 40 (22.9) | 135 (77.1) | 15 (9.1) | 149 (90.9) | 0.001 |
| Ceftriaxone | 6 (7.6) | 73 (92.4) | 20 (11.4) | 82 (89.1) | 12 (7.3) | 152 (92.7) | 0.447 |
| Carbapenems | |||||||
| Imipenem | 17 (18.9) | 73 (81.1) | 34 (19.4) | 141 (80.6) | 27 (16.2) | 140 (83.8) | 0.720 |
| Meropenem | 17 (18.7) | 74 (81.3) | 24 (13.7) | 151 (86.3) | 25 (14.9) | 143 (85.1) | 0.527 |
| Aminoglycosides | |||||||
| Amikacin | 20 (23.5) | 65 (76.5) | 58 (33.3) | 116 (66.7) | 32 (19.3) | 134 (80.7) | 0.011 |
| Tobramycin | 26 (31.7) | 56 (68.3) | 48 (28.4) | 121 (71.6) | 39 (24.1) | 123 (75.9) | 0.412 |
| Gentamicin | 16 (17.6) | 75 (82.4) | 50 (29.4) | 120 (70.6) | 34 (20.6) | 131 (79.4) | 0.058 |
| Trimethoprim- sulfamethoxazole | ND | ND | 40 (25.6) | 116 (74.4) | 31 (18.7) | 135 (81.3) | 0.141 |
| Fluoroquinolones | |||||||
| Ciprofloxacin | 22 (25.0) | 66 (75.0) | 49 (28.7) | 122 (71.3) | 29 (17.5) | 137 (82.5) | 0.048 |
| Levofloxacin | ND | ND | ND | ND | 8 (11.3) | 63 (88.7) | - |
| Tetracyclines | |||||||
| Tetracycline | 11 (12.2) | 79 (87.8) | 29 (17.4) | 138 (82.6) | 17 (10.4) | 146 (89.6) | 0.176 |
| Tigecycline | ND | ND | ND | ND | 28 (25.0) | 84 (75.0) | - |
Abbreviation: ND, not determined.
a Values are expressed as No. (%).
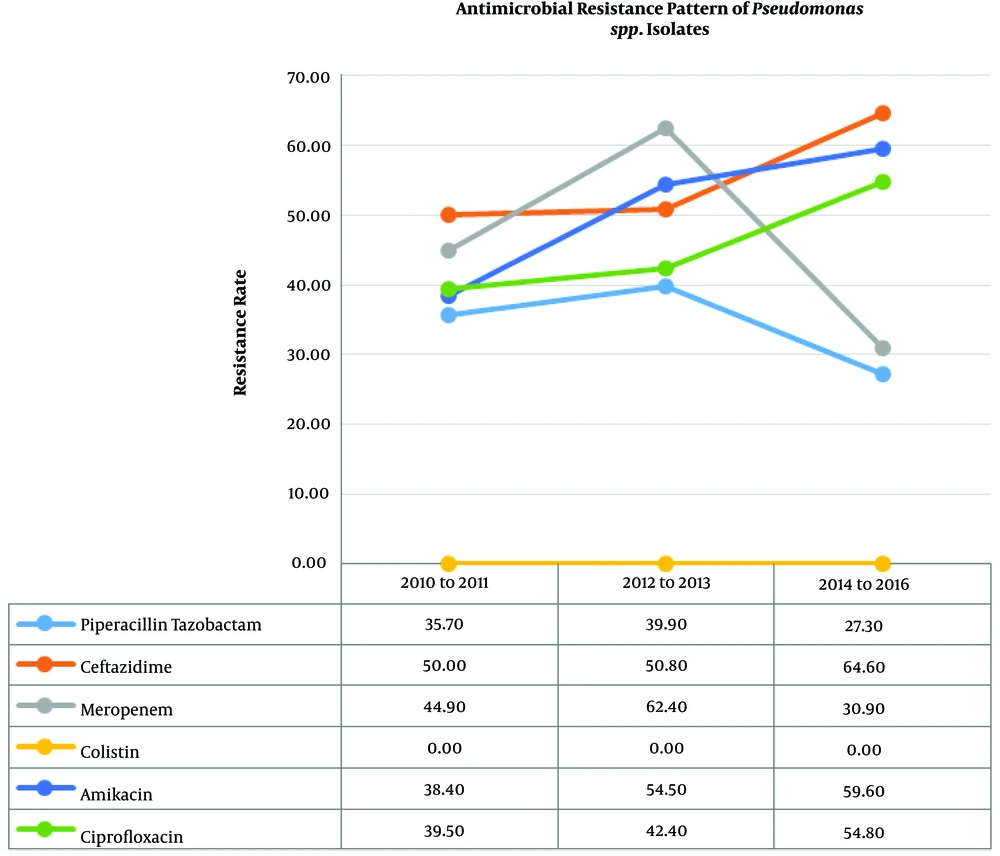
The total number of isolated Pseudomonas spp. was 473. There was a statistically significant increase in the antibacterial resistance trend of Pseudomonas spp. to amikacin (P = 0.008), tobramycin (P = 0.039), gentamicin (P = 0.003), cefepime (P = 0.008), ceftazidime (P = 0.011), and ciprofloxacin (P = 0.017). Table 3 and Figure 2 show the trend of resistance rate of Pseudomonas spp. to the antibiotics, with the resistance to colistin being zero percent throughout the three periods.
| Antibiotics | Susceptibility | P Value | |||||
|---|---|---|---|---|---|---|---|
| 2010 to 2011 (N = 90) | 2012 to 2013 (N = 194) | 2014 to 2016 (N = 189) | |||||
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | ||
| Penicillins | |||||||
| Piperacillin | 9 (30.0) | 21 (70.0) | 72 (44.7) | 89 (55.3) | 37 (50.0) | 37 (50.0) | 0.174 |
| Ticarcillin | 25 (34.2) | 48 (65.8) | 67 (36.0) | 119 (64.0) | 42 (44.7) | 52 (55.3) | 0.292 |
| Beta-lactam/beta-lactamase inhibitors combinations | |||||||
| Piperacillin-Tazobactam | 36 (64.3) | 20 (35.7) | 116 (60.1)) | 77 (39.9) | 136 (72.7) | 51 (27.3) | 0.031 |
| Cephalosporins | |||||||
| Cefepime | 33 (39.8) | 50 (60.2) | 73 (40.8) | 106 (59.2) | 49 (26.3) | 137 (73.7) | 0.008 |
| Ceftazidime | 44 (50.0) | 44 (50.0) | 94 (49.2) | 97 (50.8) | 67 (35.4) | 122 (64.6) | 0.011 |
| Monobactams | |||||||
| Aztreonam | 23 (34.3) | 44(65.7) | 41 (21.5) | 150 (78.5) | 40 (21.6) | 145 (78.4) | 0.085 |
| Carbapenems | |||||||
| Imipenem | 51 (58.0) | 37 (42.0) | 88 (46.3) | 102 (53.7) | 133 (70.4) | 56 (29.6) | < 0.001 |
| Meropenem | 49 (55.1) | 40 (44.9) | 71 (37.6) | 118 (62.4) | 130 (69.1) | 58 (30.9) | < 0.001 |
| Lipopeptides | |||||||
| Colistin | 22 (100.0) | 0 (0.0) | 143 (100.0) | 0 (0.0) | 178 (95.7) | 0 (0) | - |
| Aminoglycosides | |||||||
| Amikacin | 45 (61.6) | 28 (38.4) | 86 (45.5) | 103 (54.5) | 76 (40.4) | 112 (59.6) | 0.008 |
| Tobramycin | 32 (48.5) | 34 (51.5) | 64 (33.9) | 125 (66.1) | 58 (31.0) | 129 (69.0) | 0.039 |
| Gentamicin | 47 (52.8) | 42 (47.2) | 71 (38.2) | 115 (61.8) | 57 (31.0) | 127 (69.0) | 0.003 |
| Fluoroquinolones | |||||||
| Ciprofloxacin | 52 (60.5) | 34 (39.5) | 110 (57.6) | 81 (42.4) | 84 (45.2) | 102 (54.8) | 0.017 |
| Levofloxacin | ND | ND | ND | ND | 33 (41.3) | 47 (58.8) | - |
Abbreviation: ND, not determined.
a Values are expressed as No. (%).
5. Discussion
In this study, we surveyed the antimicrobial resistance patterns of Acinetobacter and Pseudomonas spp. isolates causing BSIs in Shiraz, southern Iran, over a seven-year period. The main finding of this study is the growing trend of antimicrobial resistance of Acinetobacter and Pseudomonas spp. to the most popular antimicrobials, e.g., aminoglycosides, fluoroquinolones, and third-generation cephalosporins. Moreover, colistin was found to be the most effective antibiotic against Acinetobacter and Pseudomonas spp. Acinetobacter and Pseudomonas spp. are recognized for their capability to develop resistance rapidly against most antibiotics that is why knowledge of the best antibiotic against these pathogens is very crucial.
The effect of ciprofloxacin on Pseudomonas spp. (sensitivity of 45.2%) was relatively superior to the effect on Acinetobacter spp. (sensitivity of 17.5%); however, for both organisms, there was a statistically significant increase in the rate of resistance to ciprofloxacin over the seven-year period. A previous report from our center showed that 83% of Pseudomonas spp. were sensitive to ciprofloxacin within 2005 - 2006 (12), indicating a growing trend. The resistance rate to levofloxacin, which was determined only in the last episode, was more than that to ciprofloxacin. Carbapenem has been the treatment of choice for the infections caused by MDR Gram-negative bacilli, but unfortunately, the resistance to carbapenem compromises the treatment options. The results of the present study indicate that Acinetobacter spp. had a high resistance rate to this group of antibiotics in 2014 - 2016. A study from southern Iran demonstrated oxacillinases (OXA-type β-lactamases) had become a principal carbapenem resistance determinant in Acinetobacter baumannii clinical isolates (2).
The resistance of A. baumannii to carbapenems was reported as 23% in 2007 - 2008 in our center (13). At present, nearly 92% - 76% of Acinetobacter isolates are resistant to imipenem in different regions (14). Our results revealed that a statistically significant decreasing trend exists for the resistance of Pseudomonas spp. to both imipenem and meropenem (29.6% for imipenem and 30.9% for meropenem in 2014 - 2016). These results were similar to the resistance rate of Pseudomonas spp. to imipenem in our center from 2005 to 2006, which was 23% (12).
We did not investigate the exact cause of the diminished resistance to carbapenems, but previous studies propose some mechanisms to explain how resistance rate of Pseudomonas spp. can be reduced. Controlling the use of one antibiotic can lead to a significant decrease in the rate of resistance (15). The restriction to ciprofloxacin at a large teaching hospital in the United States revealed a decline in the percentage rate of P. aeruginosa resistance to ciprofloxacin, carbapenems, and cefepime (16). Another study reported a significant decreasing trend in the resistance rate of P. aeruginosa, isolated from wound swabs, to ciprofloxacin, ceftazidime, meropenem, and imipenem. They suggested that it can be due to the reduction in the use of ciprofloxacin (17). Another study from China reported the same trend for the resistance of P. aeruginosa to carbapenems. The rate of resistance decreased during 2006 - 2014 (18). Exposure to ciprofloxacin causes selective mutations that upregulate the MexEF-OprN efflux system, decrease the levels of outer membrane porin protein D (OprD), and cause resistance to both fluoroquinolones and carbapenems (19).
Therefore, one of the possible reasons for the reduction of resistance is the decrease in the prescription of imipenem and meropenem in the last episode for the treatment of patients with suspected MDR Pseudomonas spp. BSIs, because of the high resistance rate between 2012 and 2013. We could not demonstrate any association between the use of carbapenems and resistance to it because this study was designed retrospectively and the data regarding antibiotics usage were lacking in our centers. In our study, more than 90% of Acinetobacter and Pseudomonas spp. were ESBL producers, which explains resistance to cephalosporins, penicillins, and monobactams (20). In a previous report from our center, among the organisms isolated from hospitalized patients, the ESBL producer rates were 81.4% (70 of 86) for Acinetobacter spp. and 71.7% (71 of 99) for Pseudomonas spp. (21).
Colistin, with proven efficacy in the treatment of bloodstream, urinary tract, and wound infections caused by Acinetobacter and Pseudomonas spp., belongs to lipopeptide antibiotics. In our study, the most effective antibiotic against Acinetobacter and Pseudomonas spp. was colistin. The resistance rates of Acinetobacter spp. to tetracycline and tigecycline, a minocycline derivative, were high (89.6% and 75%, respectively). An investigation from China on 121 Acinetobacter spp. demonstrated a tigecycline susceptibility rate of 74.5% (22). Although the prescription of tigecycline was not listed in the pharmacopeia of the hospitals of this study, the rate of resistance was high, which could be explained by the cross-resistance by multisubstrate efflux pump (23). This can explain the high rate of tigecycline resistance in our center among Acinetobacter spp.
The present study has some limitations such as the lack of clinical data, as it was a retrospective laboratory-based study. According to the CLSI guideline, the disk diffusion method is accepted as a standard for reporting the resistance rate of Acinetobacter and Pseudomonas spp. to the reported antibiotics. The only exception is concerned with the resistance rate of Acinetobacter spp. to colistin. The disk diffusion method should be used cautiously to determine the resistance rate of Acinetobacter spp. to colistin. Since the present study focused on the two abovementioned pathogens, there might have been other pathogens causing BSI with an increasing trend of resistance.
5.1. Conclusions
According to the results, the resistance of Acinetobacter and Pseudomonas spp. to almost all antibiotic classes was high and increasing over the seven-year period of this study. Nowadays, a limited number of effective antibiotics are available for empirical therapy against Acinetobacter and Pseudomonas spp. The results emphasize the need for developing and intensifying infection control programs and antibiotics stewardship for proper prescription of antibiotics in hospitals to curb the increasing trend of resistance.