1. Background
One of the most frequent infectious diseases all around the world is urinary tract infection (UTI) (1, 2). Also, the most frequent hospital-acquired infection in humans is UTI and approximately 150 million cases are recorded yearly worldwide (3). Uropathogenic Escherichia coli (UPEC) is one of the most significant causes of UTI and is responsible for 70% - 90% of the urinary tract infections (4). Uropathogenic E. coli could resist against some of the host immune factors like urine flow, antimicrobial molecules in urine and the host immune response upon gaining access into the urinary tract (5). Uropathogenic E. coli has evolved a number of mechanisms to fight against these host immune responses and factors. Attachment and invasion of UPEC to the urinary tract epithelial cells are one of the mechanisms adopted and is the first essential step taken towards establishing an effective UTI.
The result is pathogen colonization of host tissue, and this colonization causes escaping from host defenses and a reservoir for recurrent infection comes to existence. In the E. coli strains especially the strains that are important in cystitis, pyelonephritis or prostatitis, the most frequent virulence factor encoding gene is the gene for type 1 fimbriae, and it is essential for bladder colonization and there is 89 to 95% rate prevalence for it (1). These organelles are peritrichous adhesive parts that have FimA subunits and every subunit is a 7-nm wide rod part that is linked to a short 3-nm-wide distal tip fibrillum structure makeup of FimF and FimG that are the adapter subunits. The mannose-binding adhesion in UPEC isolates that effectively bind both monomannose and trimannosecontaining glycoprotein receptors are FimH. Studies have shown that neutralization of FimH with antibodies or disruption of the fimH adhesion gene is the effective methods for the impairment the colonize ability of UPEC in the urinary tract, especially the bladder (6). The fim have shown the highest distribution of virulence genes in UPEC strains isolated from UTIs patients in the studies that have assessed the UPEC in Iran (7).
Antibiotics are used to control and treat UTIs. However, the continuous usage of antibiotics for the treatment of UTIs has resulted in the emergence of antibiotic-resistant UPEC (8, 9). Thus, we could not prevent all UTIs by prescribing antibiotics and, therefore, proposing effective alternative strategies is a necessity for the decrement and prevention of UPEC infections. Nanotechnology is used for the immunization, design, and delivery of antimicrobial drugs, diagnosis, and cross-infections control, particularly at the field of overcoming antibiotics-resistant pathogens. This technology has been assessed and proposed as a superseded for the current antibiotics-based treatments of infectious disease such as UTI. Antimicrobial nanoparticles (NPs) like zinc oxide nanoparticles (ZnO) show many unique advantages that include lowering severe toxicity, control the resistance, and revealing cost-effective drugs in the future (10, 11). It is shown that ZnO NPs have antibacterial activity and anti-adhesive against important foodborne pathogens, such as E. coli O157:H7, enterotoxigenic E. coli and other pathogens (12, 13). Cell culture methodologies are used as experimental techniques for surveying the adherence/anti-adherence characteristics of E. coli strains that are related to UTIs (14, 15).
2. Objectives
This study should be served as a beginning, and there is a need for more research to absolutely determine the anti-adhesion activity of the nanoparticles suspensions against UPEC in cell cultures. Generally, assessing the anti-adhesion activity of the ZnO NPs against UPEC in T24 cell cultures is considered in this study. We also studied the effect of ZnO on the fimH gene expression in UPEC by using RT-qPCR.
3. Methods
3.1. Preparation of ZnO Suspensions
The ZnO - nano powder (approximate particle size of 10 to 30 nm; specific surface area: 19 ± 5 m2/g, Purity > 99%) was purchased from the US Research Nanomaterial Co. (USA, lot number: us3590). Afterward, the ZnO NPs powder was mixed with sterile propylene glycol. After that, for preventing the agglutination of the nanoparticles, the sonication method was used for 30 minutes at a frequency of 20000 Hz. Eventually, nanoparticles solutions with different concentrations were prepared by using this solution.
3.2. Escherichia coli Strains and Growing Conditions
Two UPEC isolates that were isolated from patients and a standard strain (PTCC1399) were used in this study. These E. coli strains all expressed type1 fimbria that is mentioned as an important virulence factor in the pathogenesis of UTI and interference in the adhesion to the epithelial cells. These strains were stored frozen at -70°C in a culture medium consisting of the sterilized mixture of glycerol (20% v/v). The solutions that contain the frozen bacteria were cultured in the mediums for overnight and at 37°C. These overnight cultures were used to obtain 1.5 × 108 CFU.mL-1 bacterial solutions that were used for adherence assays.
3.3. Antibacterial Activity Assay
The minimum inhibitory concentration (MIC) of ZnO NPs was determined by Agar dilution method (16, 17). Mueller Hinton agar mediums were mixed with different ZnO NPs concentrations. Afterward, a 0.5 McFarland standard suspension of each strain that was prepared before was inoculated on the media. The incubation of the plates was at -37°C for 24 hours. The lowest concentration at which NPs suspensions totally prevented the growth of isolates was determined as MIC, and concentrations lower than the determined MIC were considered as sub-MIC concentrations.
3.4. Cell Culture
The uroepithelial cell line derived from transitional bladder carcinoma named T24 cells (ATCC®HTB4TM) were used in this study. It has been shown that this cell line is similar to primary human bladder epithelial cells (18). The medium that was used for growing and maintaining T24 bladder cells was RPMI 1640 cell culture medium (Sigma-Aldrich, USA) and by adding 10% (v/v) fetal bovine serum at 37°C in a 5% CO2/95% air atmosphere and persistent humidity, a better condition for cells was prepared. In the experiments, for seeding the cells, 24-well tissue plates were used and for cell attachment and to obtain a cell monolayer in the plates, we waited for nearly 24 hours.
3.5. Cytotoxicity Assay/Cell Viability
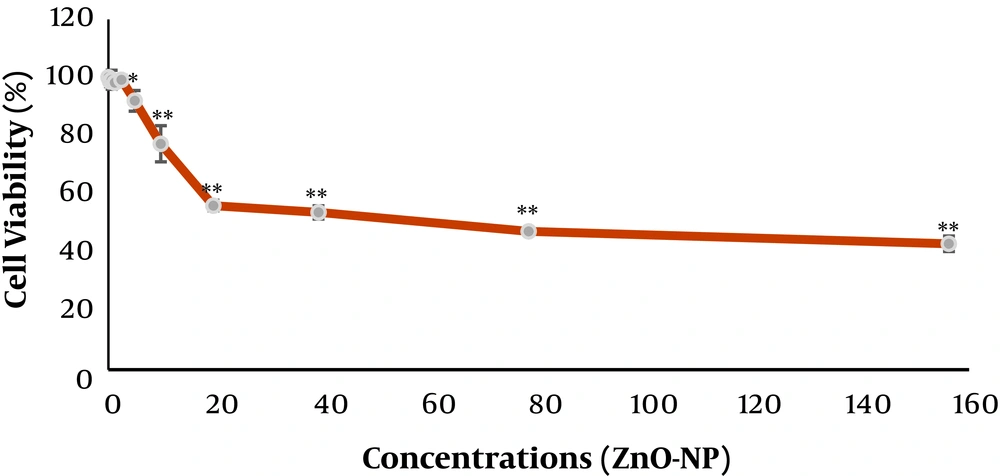
The colorimetric 3-[4, 5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay was used for assessing the cytotoxicity of the ZnO NPs suspensions opposed to the cells. In MTT assay, the reduction of the MTT dye to formazan (an insoluble intracellular blue product) occurs and the dehydrogenases of cells reduce the dye. In briefly, at first, the seeded T24 cells in plates (24-well) were incubated with suspensions for 24 hours. Then the supernatant was moved away, the Dulbecco's Phosphate-Buffered Saline (DPBS) washing of the monolayer was done, and MTT (0.5 mg.mL-1) was added to each well and left to incubate for another 3 hours at -37°C. The resulted absorbance was measured with a plate reader at 570 nm. The assessments were run for three times. The cell viability (percentage of control) was considered as the ratio of absorbance between cell culture that was treated with the suspensions and the untreated control multiplied by 100.
3.6. Anti-Adherence Assay
The inhibition of UPEC attachment to the T24 monolayer cells by ZnO NPs suspensions was determined by plate counting. Uropathogenic E. coli overnight cultures were used for the preparation of bacterial suspensions (1.5 × 108 CFU.mL-1). The bacterial suspensions were incubated for 1 h at -37°C (agitation; 180 rpm). Then, DPBS solution was used for washing the T24 cell monolayers that were confluent (105 cells/well) and the pre-incubated UPEC bacterial suspensions were added together by the ZnO NPs suspensions or DPBS (as control). After the period of incubation (1 hour) at -37°C under 5% CO2 atmosphere, for removing the unbound bacteria, wells were softly washed with Sterile DPBS solution. Cells and adhered bacteria were then detached by adding Triton X-100, 0.1% for 2 minutes to lyse the cells and isolate bacteria (19). Serial dilution plate method in BHI plates was the method that was used for bacterial counts (CFU.mL-1). The assessments were done for three times, and two times repeat was done for the experiments. The percentage (%) of the bacterial adherence was estimated by the number of adhered bacteria (CFU.mL-1) relative to the total number of bacteria added initially × 100. The percentage of inhibition by ZnO NPs suspensions was estimated as [1 - (% adherence sample/% adherence control)] × 100 (4).
3.7. FimH Gene Expression
ZnO effect on UPEC gene expression that is encoding FimH was investigated by using real-time PCR. RNX-Plus kit (SinaColon Co.) was used for total RNA extraction, by considering the manufacturer’s instructions. Also, genomic DNA was eliminated by RNase-free DNase I (Thermo Scientific), the instructions of the manufacturer were considered. The synthesis of cDNA was done by RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). The cDNA was used as the template for assessing FimH gene expression. The specific primers were used (Table 1). Real-time PCR was conducted. The ABI-7300 system (Advanced Biosystems, Foster, California, USA) and SYBR Green kit (Ampliqon, Denmark) were used for the process. The assessments were carried for RNA samples of two separate cultures of UPEC isolates.
3.8. Statistical Analysis
To explain the data, the mean and standard deviation were used. The data were analyzed using one sample Bootstrap and two sample Bootstrap methods. All analyses were performed using SPSS software version 22.
4. Results
The MIC was determined as 1250 µg.mL-1 for all three strains that were used in this study. The results of MTT assay that were used for assessing the toxicity of the ZnO NPs for the T-24 cells were depicted in Figure 1. The results of the adhesion assay that were carried out by the described method are shown in Table 2.
| Bacterial Strains | Anti-Adherence (%) in Different ZnO NPs Concentrations (Mean ± SD) | Mean Difference | BCa 95% CI | |
|---|---|---|---|---|
| 0.3 (µg.mL-1) | 19.53 (µg.mL-1) | |||
| Strain 1 (1399) | 28.777 ± 4.912a | 29.702 ± 18.451a | ||
| Strain 2 (320) | 21.030 ± 6.685a | 36.227 ± 8.071a | 15.197 | (7.859, 23.155)b |
| Strain 3 (299) | 36.455 ± 8.183a | 44.718 ± 5.100a | 8.263 | (-2.064, 16.368) |
aMean significantly different from zero using one sample Bootstrap method based on one sample BCa 95% of mean (not shown).
bThere is a significant difference between the two groups using the two sample bootstrap method based on the two sample BCa 95% CI of the mean difference between two groups.
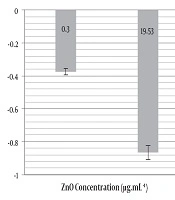
Real-time PCR results revealed that in the UPEC 1399 strain treated with 19.53 and 0.3 µg.mL-1 ZnO nanoparticle concentrations compared to the control, the expression of fimH was down-regulated by 0.865 and 0.375-fold respectively (Figure 2). The decreased expression of the fimH gene was not significant in real-time PCR assay (P = 0.203).
5. Discussion
Urinary tract infection is mentioned as an important problem related to human health and health care industry all over the world. Uropathogenic E. coli is one of the most important etiologic agents of UTI (22). Known UPEC virulence factors including type I, P and S/F1C related pili adhesions show an important interference in UPEC attachment and invasion to the host cells. FimH protein is the major units of type I pili encoded by the fimH gene. In several studies, it has been shown that in UPEC an important virulence factor is type 1 pili that interference in the colonization of the urinary tract. By FimH neutralization with antibodies or fimH adhesion gene disruption, UPEC potential for colonization the urinary tract, especially the bladder is impaired considerably (1, 23, 24).
There are various antibiotics for the treatment of UTI; however, the ability of UPEC to adhere to epithelial cells and form biofilm as well as the materializing antibiotic resistance in UPEC isolates; emphasize the necessity of proposing secure, effective alternative approaches to control UPEC infection (1). Thus, for this reason, NPs are under study today. Shakerimoghaddam et al. (25) showed that ZnO NPs could decrease the biofilm formation of UPEC significantly. As earlier noted biofilm formation by UPEC is necessary for bacterial colonization and prior to this, UPEC must adhere to the surface of cells. They also have shown that ZnO NPs could decrease the flu gene expression in UPEC isolates which is a major virulence factor of UPEC.
Therefore this study assessed the effect of ZnO NPs on the adhesion of UPEC to the T-24 cells by a cell culture method. Real-time PCR was used to investigate the inhibitory effect of ZnO-nanoparticles on the fimH gene expression; fimH gene is the critical gene that interference in the attachment and invasion of UPEC to the host tissue. The MTT assay results in this study are consistent with the results of Akhtar et al.’s study (26). The results of Agar dilution method for the determination of MIC for UPEC strains are consistent with the results of Shakerimoghaddam et al. (25). The result of this study has shown the decreased adherence of UPEC to the T-24 cell by adding a determinate concentration of ZnO NPs suspensions that are non-toxic (0.3 µg/mL) or semi-toxic (19.53 µg/mL) for the T24-cells. We must know that these NPs have more toxic effects on the cancer cell lines than normal ones (26-28). Thus, using normal cell lines will be important for future studies. As we have seen in our results ZnO NPs by 0.3 µg.mL-1 are not toxic for T24 Cells.
The T24 cell line is a human cancer cell line that is fully similar to normal human epithelial urinary tract cells. However as Akhtar et al. (26) have shown, the ZnO NPs are more toxic for cancer cell lines compared to the normal cell lines. They have shown that by using suspensions with the 15 μg/mL concentration, 33%, 39%, and 47% decreases in cell viability was seen for the BEAS-2B, HepG2, and A549 cells, respectively; which is a more toxic effect of ZnO NPs compared to normal cell lines. The lower concentrations are not toxic for these cells. So we must use a lower concentration of ZnO NPs suspensions in this study for cell culture method assay. A normal cell line with the upper concentration of ZnO NPs suspension, if applied, can show a more protective effect against the attachment of UPEC to the T24 cells and the fimH gene expression. However, the effects of ZnO NPs on modulating virulence genes expression in UPEC are essential to fully explain the molecular mechanisms in the attachment and invasion inhibition of host tissue.
5.1. Conclusions
The MIC for the three UPEC strains that were used in this study was 1250 µg.mL-1 such that when compared to the IC50 of ZnO NPs for the T24-cells (19.53µg.mL-1) is observed that have a high concentration. However, the low concentrations of ZnO suspensions that were used showed a 28.77 to 44.71% decrease in the attachment of UPEC to the T24 cells and also these low concentrations of ZnO could decrease the expression of the fimH gene in the UPEC strain. This study has shown the anti-adhesive effect of ZnO suspension against UPEC adhesion to the T24 cells and the decreased fimH gene expression are seen in these low concentrations of ZnO suspensions.