1. Background
Streptococcus pneumoniae was recognized as the main cause of community-acquired pneumonia, tympanitis, meningitis, and abscess in 1881, especially in infants and the elderly (1). Approximately, one million children under the age of five years succumb to pneumonia annually worldwide with S. pneumoniae being a major cause of death (2, 3). Streptococcus pneumoniae also thrives in the upper respiratory tract of asymptomatic carriers as an opportunistic pathogen (4). However, it is difficult to predict when it turns pathogenic. This is because the transition from a primary colonization bacterium to a pathogenic bacterium involves a complex interaction between S. pneumoniae and the body's immune system. There are various components associated with S. pneumoniae-induced immune response, such as the capsule and other virulence factors (5-7). In addition, many immune cells such as neutrophils, monocytes/macrophages, and dendritic cells are involved in this process (6, 8-10), which are responsible for the release of factors including IL-1α, IL- 1β, IL-6, IFN-α, IL-8, and ICAM-1 (6, 8).
The incidence of S. pneumoniae infection-induced septicemia and meningitis is about 5%, and its highest mortality exceeds 30% (11, 12). There is limited research on the blood and nervous system invasion by S. pneumoniae, which may be attributed to difficulties in composing clinical cases. As indicated earlier, S. pneumoniae infection inevitably involves the interaction between the body’s immune system and virulence factors.
A549 cell is a lung cell derived from human lung adenocarcinomas. Lung cells are important defense cells against S. pneumoniae infections, and are the most commonly used cells in studying the pathogenicity of S. pneumoniae. The adhesion property of different S. pneumoniae strains has been verified using A549 cells (13). Most studies on S. pneumoniae tried to focus on individual cytokines or individual virulence genes (14). However, many virulence genes may play combined roles in the S. pneumoniae invasion process and final pathogenesis.
2. Objectives
Since the interactions of S. pneumoniae and the body are very complex, this study selected a representative cytokine along with an adhesion molecule to represent the interaction. In the present study, we used different sources of S. pneumoniae to stimulate A549 cells and analyze the changes in the secretion of cytokine IL-8 and adhesion molecule sICAM-1. We also attempted to determine the characteristic biological differences of S. pneumoniae concerning source differences and explore the possible mechanisms to answer how S. pneumoniae invades blood systems.
3. Methods
3.1. Strains and Cells
Streptococcus pneumoniae ATCC 49619 was provided by the National Center for Medical Culture Collection of China. Twenty-three S. pneumoniae clinical non-repetitive strains were obtained from the Second Affiliated Hospital of Wenzhou Medical University in 2009. Eleven strains were isolated from blood samples, which were denoted as blood-derived S. pneumoniae (bd-SP). Twelve strains were isolated from sputum samples, which were denoted as sputum-derived S. pneumoniae (sd-SP). All the strains were identified using a VITEK-32 GPI card (bioMerieux, France) and confirmed by a specific PCR assay (15). A549 cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
3.2. A549 Cell Culture and Collection
The A549 cell line was cultured in F-12K nutrient mixture (GIBCO Co. Ltd., USA) containing 10% fetal bovine serum (Zhejiang Tianhang Biological Technology Co., Ltd. Zhejiang, China) and 1% penicillin-streptomycin (Beijing Solarbio Science and Technology Co., Beijing, China) at 37°C with 5% CO2 in an incubator (Model 311, Thermo Electron Instruments Co. Ltd., USA). A549 cells within seven generations were collected with the aforementioned medium without 1% penicillin-streptomycin. Cells were finally adjusted to 3.0 × 108 /L.
3.3. A549 Cell Stimulation Array
In this procedure, 1.0 mL of A549 cell concentrated suspension was added to 50 wells of a cell culture plate and two wells were designated as controls. Then, 100 µL of 1.0 McFarland S. pneumoniae concentrated suspension was added to each well while 100 µl of saline water was added to the control wells. Subsequently, the plate was incubated at 37°C with 5% CO2. After four hours, the contents of the first 25 wells (including one control) were transferred into 1.5-mL centrifuge tubes. Next, the tubes were centrifuged at 4,000 rpm for 10 minutes and the supernatants were used for the determination of IL-8 and sICAM-1. After eight hours, the contents of the remaining 25 wells (including one control) were taken and the above-mentioned steps were repeated.
3.4. IL-8 and sICAM-1 Detection
The detection of IL-8 and sICAM-1 was performed using the double-antibody sandwich avidin-biotin complex-enzyme-linked immunosorbent assay (ABC-ELISA) method, according to the manufacturer’s protocol (Shanghai Xitang Biological Technology Co., Ltd., Shanghai, China).
3.5. Statistical Analysis
SPSS17.0 software (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. The following criteria were checked: normality at P > 0.10 and homogeneity of variances at P > 0.10. The P values of < 0.05 were considered to indicate statistically significant differences.
4. Results
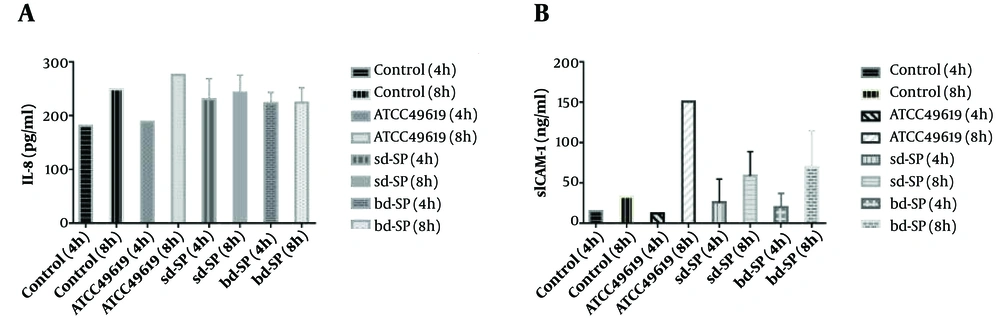
As shown in Figure 1A, A549 cells secreted higher and lower levels of IL-8 than the blank control after four and eight hours of stimulation by bd-SP, respectively. All differences were significant. After four hours of sd-SP stimulation, A549 cells secreted more IL-8. However, after eight hours of stimulation, the difference in the concentration of IL-8 was not statistically significant between the sd-SP group and blank control. Either of the clinical S. pneumoniae groups induced A549 cells to secrete more IL-8 levels than the corresponding ATCC49619 group did. However, after eight hours of stimulation, either of the S. pneumoniae groups could induce A549 cells to secrete lower IL-8 levels than the ATCC49619 group did. All differences were statistically significant. For each of the S. pneumoniae groups, there were no significant differences in the concentration of IL-8 between four and eight hours of stimulation. At the same stimulation time, there was no significant difference between the two S. pneumoniae groups in terms of the concentration of IL-8 in A549 cells.
Figure 1B shows the concentration of sICAM-1 after S. pneumoniae stimulation for four and eight hours. There was no statistically significant difference in the concentration of sICAM-1 in A549 cells between either of the S. pneumoniae groups and the blank control after four hours of stimulation. However, the differences were significant after eight hours of stimulation. Moreover, there was no statistically significant difference between either of the S. pneumoniae groups and the ATCC49619 group in the concentration of sICAM-1 in A549 cells after four hours of stimulation. However, the concentration of sICAM-1 was significantly lower in either of the S. pneumoniae groups than in the ATCC49619 group after eight hours of stimulation. The concentration of sICAM-1 significantly increased with the extension of stimulation time in the same clinical S. pneumoniae group. The peak value of sICAM-1 concentration reached after eight hours of stimulation. At the same stimulation time, there was no significant difference between the two S. pneumoniae groups in terms of the concentration of sICAM-1 in A549 cells.
5. Discussion
IL-8 is a multifunctional proinflammatory cytokine that can be secreted by numerous cells such as mesothelial cells, tumor cells, neutrophils, endothelial cells, and monocytes (8, 16, 17). The main role of IL-8 during inflammatory response is the chemotaxis of immune cells to the site of inflammation, which triggers the release of inflammatory mediators (18, 19). This process is the main response to S. pneumoniae infection, by which the host is capable of removing S. pneumoniae and maintaining homeostasis. In the present study, we found that IL-8 secretion from A549 cells significantly increased after either of sd-SP and bd-SP stimulation.
The IL-8 secretion was significantly higher in either of the sd-SP or bd-SP groups after four hours of stimulation than in the ATCC49619 positive control; however, the IL-8 secretion relatively decreased after eight hours in the stimulation groups. Furthermore, no difference in the IL-8 secretion was observed between the sd-SP and bd-SP groups. In addition, comparing the IL-8 levels between the four and eight-hour stimulations demonstrated that higher levels of IL-8 were secreted as the incubation time increased, suggesting that lung cells may produce IL-8 in both healthy and pneumonic conditions. Lung cells may also be involved in the migration of neutrophils to the lung in response to inflammation via the activation of the NF-κB pathway.
ICAM-1s are distributed to the surface of several cell types including vascular endothelial cells, epithelial cells, lymphocytes, neutrophils, and monocytes, but at low basal levels. Previous studies reported that cross-linking ICAM-1 elicits an association with the actin cytoskeleton and the activation of several intracellular signaling pathways, which triggers the production of numerous cytokines and cell trafficking (19-21). Upon S. pneumoniae invasion, host ICAM-1 may promote the leukocytes’ accumulation towards S. pneumoniae-infected sites, especially extravascular ones (22, 23). Several proinflammatory cytokines including IL-1, IL-11, tumor necrosis factor α (TNF-α), and interferon-γ (IFN-γ) are capable of enhancing the expression of ICAM-1 (24). In the present study, while IL-8 secreted by A549 cells increased and remained stable after four hours of sd-SP or bd-SP stimulation, the sICAM-1 level significantly elevated with the extension of S. pneumoniae stimulation time.
Higher levels of ICAM-1 have a positive role in the assistance of leukocyte accumulation in infectious tissues by promoting the interaction between ICAM-1 and its ligands, which is important for the host to remove foreign microbes (19, 20). It was reported that choline-binding protein A, a protein synthesized by S. pneumoniae that promotes pneumococcal adherence and the colonization of the nasopharyngeal respiratory airways, induced the release of IL-8 and ICAM-1 in A549 cells (19). ATCC49619 as reference S. pneumoniae showed an increasing trend in IL-8 secretion with increasing the duration of S. pneumoniae stimulation, whereas clinical S. pneumoniae reached the peak at four hours and remained stable thereafter. Similar trends of sICAM-1 secretions were observed in ATCC49619 and clinical S. pneumoniae. There was no significant difference in IL-8 or sICAM-1 levels between the sd-SP and bd-SP groups. However, sICAM-1 levels in A549 cells also slowly increased with incubation time but insistently at low expression levels, indicating that ICAM-1 is crucial in the immune response to S. pneumoniae infection.
There are some limitations to our study. First, many types of cell lines are involved in S. pneumoniae-host interactions and their numbers and concentrations are hard to be accurately determined. However, to simulate actual interactions, we used only one fixed concentration of A549 cells. Second, A549 cell line was derived from a lung adenocarcinoma, which, in spite of its capability of self-proliferation, was different from normal pneumocytes for antigen presentation and cytokine secretion. This study demonstrated that S. pneumoniae invasion stimulated the secretion of IL-8 and ICAM-1 in A549 cells. Despite the similar effects of sd-SP and bd-SP on IL-8 and ICAM-1 expression, significant differences between clinical S. pneumoniae and ATCC49619 in our study showed that S. pneumoniae invasion might not be predominantly caused by reactions in the host.