1. Background
Leishmaniasis is wide-spectrum disease, which is caused by the intracellular protozoan parasites of genus Leishmania (1, 2). It causes various clinical syndromes including cutaneous, mucocutaneous involvement as well as the serious visceral leishmaniasis (VL) (3, 4). Leishmania infantum and L. donovani in the Old World and L. chagasi in the New World are the causative organisms for Kala-Azar (5). It is approximated that an annual number of 500,000 cases are affected by VL globally (6). Humans, particularly children and immunosuppressed patients in Iran are mostly parasitized by L. infantum (7). Visceral leishmaniasis has been endemic in Iran and the most reported cases are from Ardabil, Fars and Bushehr provinces (4, 6, 8). Visceral leishmaniasis may manifest with various clinical features (4, 9). Accordingly, it is a requisite to base novel treatment options for this clinically important parasitic infection.
Several risk factors such as travel to endemic areas, decreased immunity, absence of an efficient vaccine enhance the occurrence of leishmaniasis (10). The primary medication for leishmaniasis mostly relies on Glucantime and Pentostam (first-line therapies) as well as Pentamidine and amphotericin B (second-line therapies). However, the long-term treatment course of this infection has invoked many concerns regarding uncommon expensive drugs, development of side effects and emergence of drug resistant parasites (11). With respect to this, the discovery of innovative drugs and/or utilization of old drugs with novel formulations against parasites are key steps towards better future medications. During the last decades, web-based software has comprehensively aided us to assess molecule-receptor interactions and discovery of new treatment options (12). More recently, the antileishmanial experiments have utilized the drugs specific for other diseases such as anticancer and anti-inflammatory compounds (13, 14). For example, doxorubicin is an antineoplastic agent which has shown to have potent effects on L. donovani (15). Moreover, considerable inhibition of L. infantum proliferation in murine model was incurred following acetyl salicylic acid (Aspirin) treatment (16).
In this case atomic force microscopy (AFM) is a high-resolution imaging to detect subtle textural alterations on cell surface. Because of its versatility, AFM has become a tool in order to investigate the intrinsic mechanical properties of cells (17, 18). By, AFM we can examine whole and subcellular compartments and as well as carry out top-down mechanical characterization (17, 19). Besides, the AFM enables us to provide images from mechanical responses of single cell to drugs or other compounds (17, 20). We used AFM to investigate the Leishamnicidal properties of ethinyl estradiol and tolmetin on promastigotes and axenic amastigotes of L. infantum. Previously, we contrived an in silico investigation of 3358 compounds and deduced that ethinyl estradiol as a contraceptive with anticancer activity and tolmetin as a nonsteroidal anti-inflammatory drug (NSAID) are the appropriate drugs for antileishmanial assay. Accordingly, we designed an experiment to assess the antileishmanial activity of ethinyl estradiol and tolmetin on the promastigotes, axenic amastigotes and macrophage-dwelling amastigotes of L. infantum using (MTT) and AFM microscopy methods.
2. Objectives
In this study, we investigate the effects of ethinyl estradiol and tolmetin on promastigotes and amastigotes of L. infantum with MTT assay and morphological changes induced by these two drugs by AFM microscopy.
3. Methods
3.1. Experimental Drugs
Regarding our previous in silico investigation, two drugs, comprising tolmetin as NSAID and ethinyl estradiol as a contraceptive having antitumor function were selected. These drugs were obtained from Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences.
3.2. Parasite Cultivation
A standard strain of L. infantum parasites (MCAN/IR/96/LONDON49) provided from Pasteur Institue of Iran. In brief, RPMI 1640 medium enriched by 10% heat deactivated fetal bovine serum (FBS) (Gibco, USA) 2 mM L-glutamine, 100 U/mL and 100 mg/mL streptomycin was used to culture 5 × 105 promastigotes per ml in a 24 ± 1ºC incubator (Shimaz, Iran). All chemicals were obtained from a Sigma (Sigma, Chemical Co., St. Louis, MO, USA). Subculture procedure was also done each 96 h at 2 × 107 cell densities/mL (10).
3.3. Promastigote Assay
The 96-well culture plates were seeded by a density of 105/100 µL L. infantum promastigotes. In addition, two control wells, one containing drug solvent and the other encompassing 40 µg/mL amphotericin B were considered. Subsequently, a 100 µL volume of various concentrations of tolmetin (200, 100, 50, 25, 12.5 and 6.25 µg/mL) and ethinyl estradiol (250, 125, 62.5, 31.25, 15.62 and 7.81 µg/mL) were added to respective wells, the examination was done in triplicates in 24 ± 1ºC incubation condition. Parasite count was performed following 24 h, 48 h and 72 h time periods using a Neubauer chamber slide. Next, 20 µL of prepared and filtered MTT solution was added to each well and incubation for 3 h at 24 ± 1ºC was carried out. After centrifugation (Sigma, Germany) the supernatant was discarded and 100 µL of dimethyl sulfoxide (DMSO) appended to all wells. Following shaking, the rate of absorbance was read at 570 nm using an ELISA reader device (Elx800, Bio Tek, USA). The percentage of viable promastigotes was calculated by the following equation:
Viable promastigotes (%) = (AT - AB) / (AC - AB) × 100
AC, AT and AB are the mean absorbance of untreated, treated and blank wells. Finally, the linear regression analysis was used to calculate IC50(10).
3.4. Amastigote Assay
In order to obtain axenic amastigotes, L. infantum promastigotes were cultured in RPMI 1640 medium with reduced pH (4.5 - 5.5), supplemented with heat deactivated fetal bovine serum (FBS), L-glutamine, penicillin and streptomycin, under 5% CO2 aeration and 37ºC incubation (Memmert, Germany). A number of 5 × 104 amastigotes were placed in 96-well culture plate. Control wells were also considered as previously. One hundred µl of different concentrations of ethinyl estradiol and tolmetin were incorporated into respective wells. Then, triplicate plates were incubated for 24, 48, and 72 hours at 37ºC. Then, the MTT assay was performed as previously described to determine the effect of ethinyl estradiol on amastigotes of L. infantum. Finally, viable amastigote count was calculated using the aforementioned formula (21).
3.5. Macrophage-Dwelling Amastigotes Assay
The macrophage-dwelling amastigotes model was used to evaluate the leishmanicidal activity of ethinyl estradiol, Accordingly, RAW 264.7 macrophages were seeded at a density of 3 × 106 cells/well into a 24-well culture plate, and allowed to adhere following incubation at 37ºC and 5% CO2. After 24 h incubation, the cells were washed to remove the unattached cells. Then, the macrophages were infected with metacyclic promastigotes of L. infantum at 10:1 (promastigote: macrophages) ratio. Further incubation was performed for 24 h at 37ºC and 5% CO2, and then the infected cells were washed to remove un-phagocytosed promastigotes. Afterwards, infected macrophages were treated with different concentrations of ethinyl estradiol for a period of 72 h. Then, the MTT assay was performed as previously described to determine the effect of ethinyl estradiol on macrophage-dwelling amastigotes of L. infantum (21).
3.6. Atomic Force Microscopy
Seventy-two hours following ethinyl estradiol challenge and incubation, cultured promastigotes and axenic amastigotes were centrifuged, then the precipitated, washed, fixed and dried. Ultimately, AFM imaging of the treated and prepared promastigotes and amastigotes was performed by an atomic force microscope (AFM Workshop, Signal Hill, USA) (17).
3.7. Statistical Analysis
Results were analyzed finally using one-way analysis of variance (ANOVA) test and provided as the mean value of triplicate experiments ± standard deviation (SD) by linear regression. Data were analyzed using SPSS© ver.22 software.
4. Results
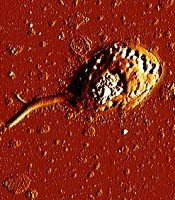
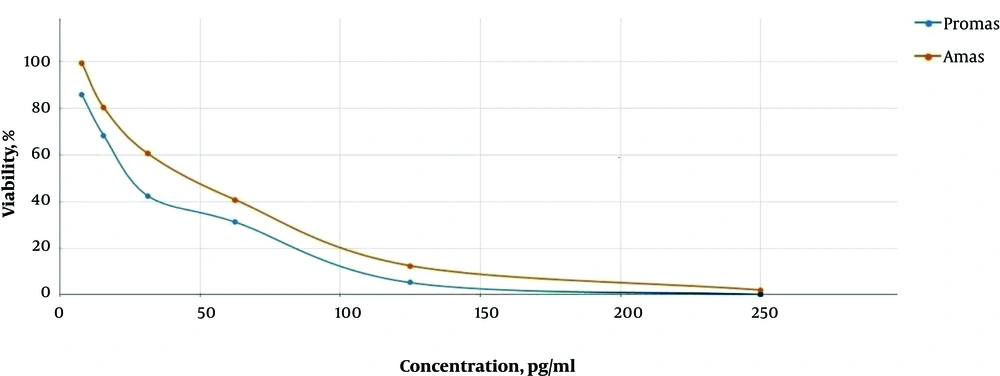
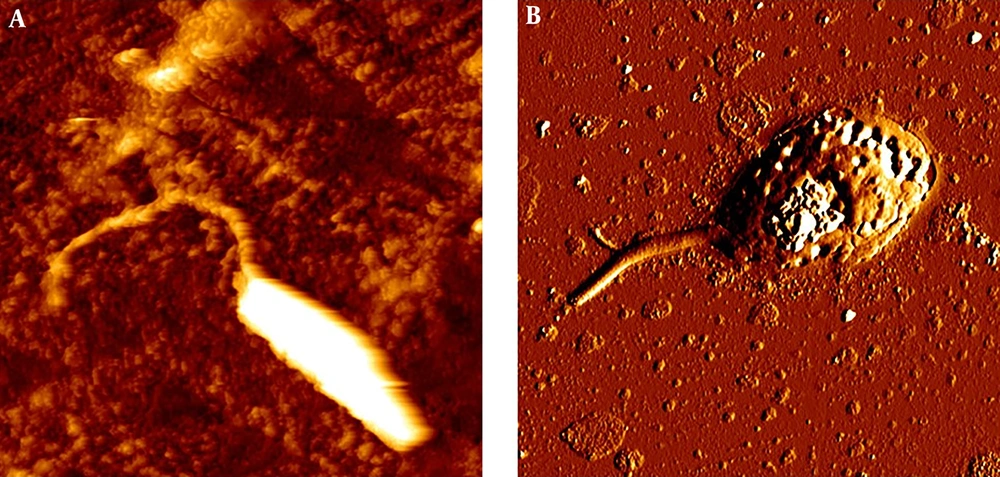
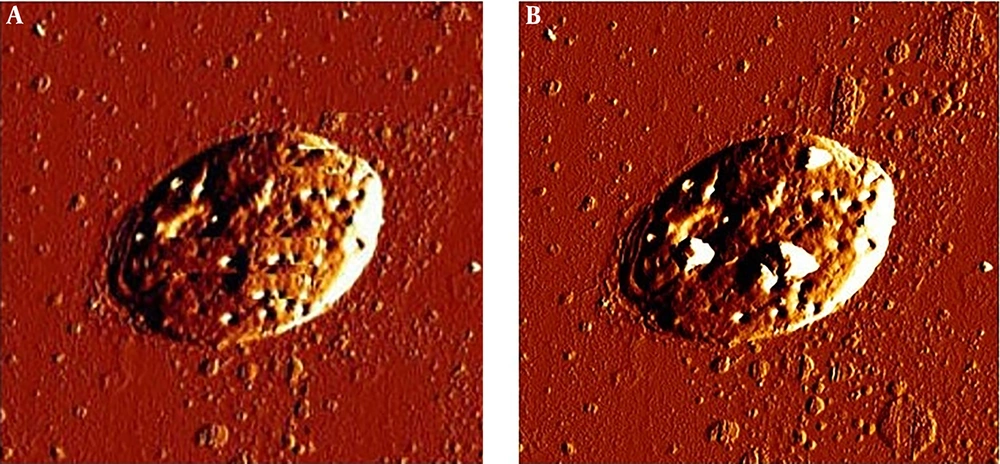
In this study the cytotoxic activity of different concentrations of tolmetin and ethinyl estradiol on cultured promastigotes and axenic amastigotes of L. infantum were evaluated for 24, 48 and 72 h in triplicate. The results of MTT test and morphology observations during 24, 48 and 72 h didn’t demonstrate a significant impact of tolmetin on L. infantum. However, ethinyl estradiol, showed statistically significant activity on both promastigotes and amastigotes during 72 h. Consequently, IC50 of this drug on promastigotes and axenic amastigotes during 72 h was 23 and 45 µg/mL, respectively (Figure 1). Moreover, AFM imaging demonstrated considerable impacts of ethinyl estradiol on morphology of L. infantum promastigotes and amastigotes. However, considering the result, it is obvious that some changes occurred morphologically (Figures 2 and 3).
The incubation of L. infantum promastigotes with ethinyl estradiol inhibited the parasite growth efficiently (Figure 1). The IC50 concentration of ethinyl estradiol and amphotericin B as control on macrophage-dwelling amastigotes was 30 μg/mL and 32 μg/mL, respectively. Our results showed that treatment of amastigotes with the different concentrations of ethinyl estradiol lead to a significant decrease (P < 0.01) of intracellular amastigotes compared with control. We assume that besides the direct actions of ethinyl estradiol, it may also act indirectly through the activation of macrophages. As illustrated in Figures 2 and 3 we investigated the effects of ethinyl estradiol on promastigotes and axenic amastigotes of L. infantum by atomic force microscopy. The analyses showed that treatment of L. infantum promastigotes and amastigotes with subjected drugs cause ultrastructural changes. These results show that the morphology of parasite was changed and the cell shape was altered.
5. Discussion
The inconvenience, toxicity and resistance issue are main complications associated with the drug therapy for the leishmaniasis (22). The VL-specific therapy mostly relies on Glucantime and Pentostam (3, 23). Toxic medications used against VL, implicating higher risk of death following treatment (23). On the other hand, leishmaniasis has co-evolved with first-line drugs beyond 70 years of administration and, to some extent, have developed novel metabolic pathways to bypass drug actions (24). Therefore, bioinformatics, can be used to revisit different drug compounds to be administered against VL (25). Khademvatan et al. showed, in silico and in vitro study could lead to find potential drugs against leishmaniasis (10).
In present study, the MTT result confirmed the antileishmanial activity of ethinyl estradiol against L. infantum. This compound is able to inhibit intracellular amastigotes growth in 50% at 30 μg/mL concentration of ethinyl estradiol similar to 32 μg/mL concentration of amphotericin B as a control. Our results show that these compounds exert similar antileishmanial effects on promastigotes as well as amastigotes. Similar to previous studies (26, 27). Our results confirmed that the ethinyl estradiol has cytotoxicity which leads to parasite destruction. Two substantial concepts support drug repositioning strategies: addressing new uses of a compound having a known target or seeking novel utilization of a drug acting via an unconventional target (28). Based on our results, ethinyl estradiol possessed dose-dependent antileishmanial effects, in contrast to tolmetin. Also, parasite count demonstrated remarkable variation between sample and control groups. On the basis of measured optical density and IC50 following 72 h, the 23, 45 and 30 µg/mL concentration of ethinyl estradiol was calculated to possess inhibitory effect on promastigotes, axenic amastigotes and intracellular parts of L. infantum, respectively.
Previously, it has been shown tamoxifen as anticancer drug has antileishmanial activity (29). Miguel et al. assessed the antileishmanial activity of an anti-estrogen drug, tamoxifen, suggesting that it can kill L. infantum promastigotes and macrophage-dwelling amastigotes (30). The cancer chemotherapy drug, cisplatin was shown to possess antileishmanial traits on L. infantum (31). The efficacy of some anticancer protein kinase inhibitors was evaluated by Sanderson and colleagues, showing sarafenib, lapatinib and sunitinib as bioactive compounds against L. donovani amastigotes in murine macrophages (32). Raloxifene, an estrogen receptor modulator, was assessed on L. donovani promastigotes and L. infantum promastigotes and amastigotes (33). Cyclosporine as an immunosuppressant agent was also tested against L. donovani (34). Moreover, in this study we find that ethinyl estradiol as an anticancer drug can kill L. infantum with the maximum effect on promastigotes. The result of atomic force microscopy showed cell alterations in promastigotes and axenic amastigots of L. infantum treated with ethinyl estradiol. In the other study with AFM showed, drugs produce severe disturbances on membrane, leading to the death of parasites (35).
Findings from other antileishmanial agents on Leishmania sps are consistent with our AFM imaging results, suggesting cell length reduction (35-37). Herein, we selected two drugs from, 3358 FDA-approved compounds with software-based analyses, i.e. ethinyl estradiol and tolmetin for assay against L. infantum promastigotes and amastigotes. Our results asserted that only ethinyl estradiol was efficacious against the parasite with IC50 scores of 23 and 45, 32 µg/mL on promastigotes, axenic and macrophage-dwelling amastigotes, respectively, after 72 h incubation. Convincing evidence from our study demonstrate that drug repositioning could be a promising, affordable alternative for better treatment of leishmaniasis. Similar to our study Khademvatan et al. showed virtual screening and drug repositioning is good strategies for the discovery of new drug against leishmaniasis (38).
In contrast to our study, Valpato et al. has used conventional electron microscopy to illustrate the effect of T6 compound on L. infantum. So, their results were not illustrative of the exact leishamnicidal activity of the subjected compound (39). In another study was conducted by Rottini et al. they were used (-)α-bisabolol as antileishmanial agent (26), however they did not provide atomic force microscopy images to clarify the morphological changes. Therefore, we strictly suggest the use of AFM, which can provide information about the how the subjected drugs can affect parasite cells. Similar to our results Valpato et al. and Rottini et al. (26, 39) have showed the leishmanicidal activity of subjected compounds, while compared to their results, our findings suggest that ethinyl estradiol can be used as a selective antileishmanial agent.
5.1. Conclusions
In this study we showed that ehinyl estradiol had good antileishmanial activity and it is recommended to be tested under in vivo condition. Furthermore, we strictly suggest the use of AFM which can provide information on how drugs can affect parasite cells.