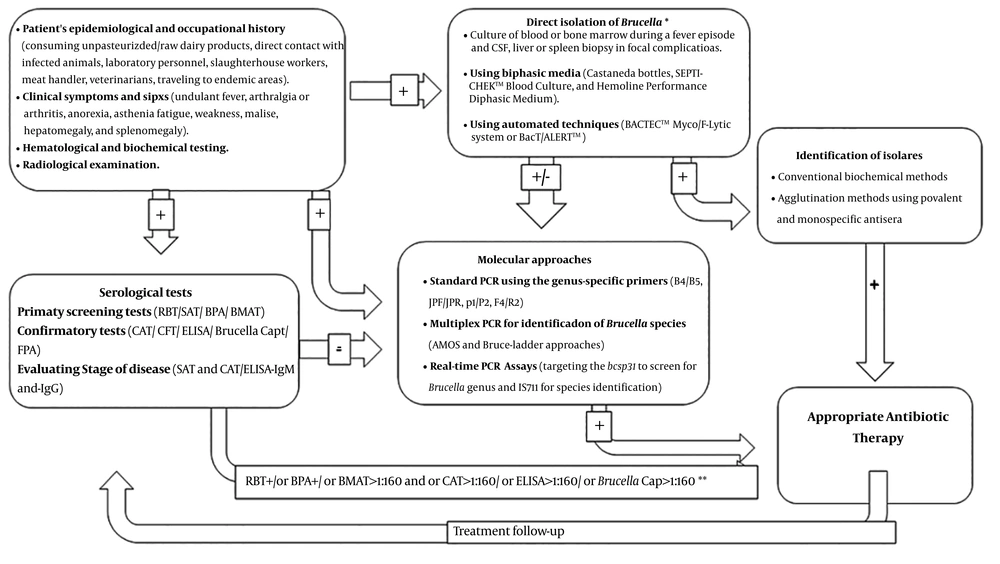
1. Context
Brucellosis is a zoonotic disease, endemic in many parts of the world especially the Middle East, South and Central Asia, Mediterranean region, Europe, North and East Africa, and Latin America with over half a million new cases annually (1-3). Clinical management of brucellosis is one of the most challenging obstacles due to a high rate of failure in treatment and subsequent relapse (4, 5). Definitive diagnosis of brucellosis needs comprehensive evaluation of the living conditions of the patient, medical history, clinical examinations, and careful interpretation of laboratory test results and radiologic findings (6, 7). Indeed, diagnosis of brucellosis is frequently delayed and often missed especially in the developing countries (8). The gold standard for diagnosis still is bacterial culture, which often fails. Thus, diagnosis relies on the combination of several methods (9).The present study aimed at reviewing laboratory tests in the diagnosis of brucellosis.
2. Evidence Acquisition
In the current review, data were retrieved by search in MEDLINE (via PubMed), Web of Science, Embase, and Cochrane databases, as well as references of the related articles. The following search keywords were used with the help of Boolean operators (AND or OR): Brucella, brucellosis, human, and diagnosis. Articles published from 1953 to December 2018 were included. Inclusion criterion was articles using the following techniques and/or methods: direct isolation and identification, conventional cultural examinations, lysis-centrifugation, blood clot culture, automated and semi-automated techniques, serological diagnostic tests, and molecular assays. After screening the abstracts in terms of applied techniques and methods, information was extracted from selected articles in terms of microbiological, serological, and molecular techniques.
3. Results
Diagnostic approaches for human brucellosis are presented in Figure 1. Comparison of different diagnostic methods for human brucellosis is described in Table 1.
| Method | Advantage | Disadvantage |
|---|---|---|
| Conventional culture | Gold standard and specificity | Time consuming, insensitive or low sensitive, and posing a risk for laboratory staff |
| BACTEC™ and BacT/ALERT™ | Rapid, sensitive, and limiting exposure to infectious agents | Costly, and need of subsequent identification |
| Serum agglutination test | Safe, inexpensive, and appropriate for primary screening | Cross-reactivity with other microorganisms, false-negative results in the early stages of infection, and prozone phenomenon |
| 2-Mercaptoethano | A confirmatory test that allows selective quantification of IgG anti-Brucella | Toxicity of mercaptoethanol, the possibility of IgG degradation by the 2-ME, which may lead to false negative results |
| Coombs antiglobulin agglutination test | Sensitive for relapsing and chronic brucellosis | Labor-intensive and time consuming |
| Rose Bengal plate agglutination test | Rapid for primary screening, simple, and inexpensive | Cross-reactivity with the antibodies of other microorganisms, false-negative results in the early stages of infection, and prozone phenomenon |
| Complement fixation test | Sensitive and specific | Complexity, high prices of reagents, need of trained laboratory technicians, and expensive equipment |
| ELISA | Highly sensitive and specific, rapid, simple, and capable of distinguishing between acute and chronic stages | Cross-reactivity |
| Fluorescence polarization immunoassay | Highly sensitive and specific, and capable of distinguishing between acute and chronic stages | Costly, need of trained laboratory technicians, and expensive equipment |
| Lateral flow assay | Easy, rapid, sensitive, and specific | Expensive and possibility of cross-reactivity |
| PCR | Rapid and accurate; can be performed on blood, serum, CSF, and other clinical samples; can yield positive results as early as 10 days after inoculation | Expensive equipment, genus specific Brucladder has low detection limit, and works only on pure cultures |
| Real-time PCR | Highly sensitive, specific, and rapid; can be performed on blood, serum, CSF and other clinical samples | Expensive equipment |
| MALDI-TOF MS | Highly sensitive and specific; can be performed on blood, serum, CSF, and other clinical samples | Expensive equipment |
| Immunoblot | Sensitive and specific | Cross-reactivity |
| NGS | Specific technique | Expensive equipment; need of software and complicated analysis |
Diagnostic approaches for human brucellosis. * Direct isolation of Brucella needs BSL-3 laboratory capability. ** In endemic areas, high titers (cutoff points) may be considered as a positive reaction. BMAT, Brucella microagglutination test; BPA, buffered plate antigen test; CAT, Coombs antiglobulin agglutination test; CFT, complement fixation test; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; FPA, fluorescence polarization immunoassay; SAT, serum agglutination tube test.
3.1. Direct Isolation and Identification
The isolation of Brucella spp. is considered as the gold standard technique for the diagnosis of brucellosis. The culture of Brucella is specific and allows definitive identification and typing of the isolates of Brucella spp. that is particularly valuable for epidemiological investigations (10). Sensitivity of the Brucella spp. isolation is variable depending on the culture method, type of clinical sample, stage of the disease, and history of antibiotic use (11, 12). The risk of acquiring an infection from laboratory ranges 40% to 100% and depends on various factors; e. g., exposures due to laboratory accidents and aerosolization of microorganisms during routine identification activities (13). The ability to direct isolation and culture of Brucella spp. can vary between acute and chronic manifestations. Although 50% - 80% of acute cases yield positive blood cultures, only 5% of chronic cases are culture-positive (12). In order to increase the sensitivity, multiple blood sampling should be conducted in the acute phase of brucellosis (14). The frequency of bacteremia episodes is another factor, which should be considered in terms of the time, frequency, and volume of blood collected for culturing. Use of bone marrow aspirate is more sensitive in patients who underwent antibiotic therapy, as well as the ones with a chronic form of brucellosis (15).
3.2. Conventional Culture Examinations
There are numerous available culture media in solid, broth, or biphasic forms for growing Brucella spp. isolated. Biphasic media such as the Castaneda blood culture bottles, SEPTI-CHEK™ blood culture (BD BBL®), and Hemoline performance diphasic medium (bioMerieux®) can be used to avoid subculture (15, 16). Commercially available biochemical tests such as API 20 NE® (bioMérieux®) are particularly useful for the rapid and easy identification.
3.3. Lysis-Centrifugation
LC technique is used to concentrate intracellular Brucella spp. in blood samples and consequently, increase the test sensitivity (17). The sensitivity, specificity, and positive and negative predictive values of the LC method are 100%, 87.8%, 81.6%, and 100%, respectively compared with those of the Castañeda method (17).
3.4. Blood Clot Culture
Clot culture is a more suitable choice when a second blood sample is not available. Since clot culture techniques are sensitive, simple, and inexpensive and yield earlier results, they can be settled in the areas where automated systems are far from reach. The overall mean time-to-detection of clot culture technique is approximately four days less than that of the conventional methods (18).
3.5. Automated and Semi-Automated Techniques
BACTEC™ (Becton Dickinson Diagnostic Systems®), and BacT/ALERT™ (bioMérieux®) are two frequently used systems in many laboratories that continuously monitor the growth of microorganisms by labeled CO2. The BACTEC™ Myco/F-Lytic system (Becton Dickinson Diagnostic Systems®) is also developed to improve the recovery rate of intracellular pathogens such as Brucella spp. by combined lytic activity and automation (19). Recently, the Micronaut™ semi-automated biotyping System (Merlin Diagnostika®), which facilitates the metabolization of various substrates by bacterial cells, was used for the identification of Brucella species and biovars (20).
3.6. Serological Diagnostic Tests
The serological diagnosis of brucellosis commonly relies on the confirmation of the rising titters of Brucella-specific antibodies. This is the indirect proof of infection. Serological assays are used for the primary diagnosis of infection, as well as treatment follow-up (21) (Table 1). Immunoglobulin (Ig) M isotype antibodies against the lipopolysaccharide (LPS) of Brucella spp. are the first immunoglobulins emerge after infection and are the predominant antibodies during the acute phase of the disease (22). The presence of specific IgM is considered suggestive of acute or recent infection. But, IgM antibody detection in the absence of IgG may lead to a misdiagnosis of acute brucellosis and may be a source of controversy (23, 24). However, the early IgM response might not be seen in patients infected with slow-onset strains, as well as in those appeared late in the course of the disease, or in those with relapses.
The titer of antibody should decline after an effective treatment. Otherwise, the patient should be examined for the possibility of chronic focal disease or relapse. Furthermore, the significant titers of antibody may persist for several months or even years in patients with the history of brucellosis. False positives in the determination of anti-Brucella IgM may be due to the presence of cross-reactions and rheumatoid factor. It may be difficult to distinguish between active infection and simply exposure to the bacteria without clinical relevance in endemic regions by serological methods (25, 26).
3.7. Fluorescence Polarization Immunoassay
Fluorescence polarization immunoassay utilizes molecular rotation, measuring antigen-antibody binding without the need for separation procedures. It requires one-step serum dilution, assessment of background fluorescence, addition of the labelled antigen, and finally measurement of antigen-antibody interaction (27). The accuracy of the FPA is equal or superior to other serological assays such as the complement fixation test (CFT) or the enzyme-linked immunosorbent assay (ELISA). The specificity and sensitivity of FPA for culture-confirmed human brucellosis is 98% and 96%, respectively (12, 28).
3.8. Immunochromatographic Lateral Flow Assay
Immunochromatographic lateral flow assay is a simplified version of the ELISA for the detection of Brucella-specific IgM and IgG antibodies (22). Immunochromatographic lateral flow assay is capable of identifying acute, persistent, and relapsing infections. It can also be used to monitor treatment. The sensitivity and specificity of ILFA to detect Brucella IgM and IgG in comparison with ELISA or CFT reported 96% and 99%, respectively (22). Therefore, ILFA for both Brucella IGM and IgG antibodies is a suitable method for endemic areas with limited resources (29).
3.9. Molecular Assays
Molecular methods become valuable tools for clinical diagnosis and public health surveillance purposes, as well as identification of species and subspecies (30). These techniques can be more sensitive than blood culture and more specific than serologic tests. Molecular assays can be performed on various clinical samples including serum, whole blood, cerebrospinal fluid (CSF), synovial or pleural fluid, urine, and even tissue specimens. Furthermore, they can supplement phenotypic tests (31, 32). However, direct detection of Brucella DNA in patients suspected of brucellosis may be a challenge due to the small number of circulating bacteria in the blood, especially in chronic courses or after antibiotic therapy. Moreover, the detection of Brucella DNA cannot demonstrate an active infection with viable pathogens, and thus, may not efficiently support the therapeutic decision making. The type of clinical sample, the DNA extraction method, the specific gene that is tracked, and the employed technique are factors that can influence the efficiency of molecular assays (33).
3.10. Standard Polymerase Chain Reaction
PCR can be performed to amplify and detect Brucella DNA in clinical samples or pure cultures. Previously, Navarro et al. described several advantages of using serum samples for nucleic acid amplification (34). Several single-step PCR assays are developed to amplify and detect specific genomic sequences of the genus, species, or even biotypes of Brucella. Primer pairs used to detect Brucella at the genus-specific level include the primers for sequences encoding BCSP31 (B4/B5), 16SrRNA (F4/R2), 16S - 23S intergenic transcribed spacers (16S - 23S ITS) (Bru ITS-S/Bru ITS-A), 16S - 23S rDNA interspace (ITS66/ITS279), IS711 (IS313/IS639), outer membrane proteins (omp2b, omp2a and omp31), per (bruc1/bruc5), and proteins of the omp25/omp31 family of Brucella (35-38). Specificity and sensitivity of these techniques vary depending on the sets of primers, type of clinical sample, and presence of human genomic DNA (Table 2).
| PCR Technique | Primer Name | Primer Sequence | Amplicon Size, bp | Annealing Temp, °C | No. of cycles | Specificity, % | Sensitivity, % | PPN, % | NPN, % | Detection Limit, fg | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| bcsp31a | B4 | TGGCTCGGTTGCCAATATCAA | 223 | 60 | 40 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 100; S: 97 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 98; S: 94.3 | 10 - 100 | (39) |
| B5 | CGCGCTTGCCTTTCAGGTCTG | ||||||||||
| omp2b | JPF | GCGCTCAGGCTGCCGACGCAA | 193 | 58 | 35 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 98; S: 95.5 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 96.1; S: 91.7 | 25 - 250 | (39) |
| JPR | ACCAGCCATTGCGGTCGGTA | ||||||||||
| omp2 | P1 | TGGAGGTCAGAAATGAAC | 282 | 50 | 30 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 99; S: 97 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 98; S: 94.3 | 12.5 - 125 | (39) |
| P2 | GAGTGCGAAACGAGCGC | ||||||||||
| bp26c | 26A | GCCCCTGACATAACCCGCTT | 1029 | 58 | 30 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 98.5; S: 96.5 | Bc: 100; Wb: 100; S: 100 | Bc: 100; Wb: 97.1; S: 93.5 | 20 - 200 | (39) |
| 26B | GAGCGTGACATTTGCCGATA | ||||||||||
| 16srRNA gene | F4 | TCGAGCGCCCGCAAGGGG | 905 | 54 | 35 | Bc: -d; Wb: 100; S: - | Bc: -; Wb: 53.1; S: - | Bc: -; Wb: 53.1; S: - | Bc: -; Wb: 100; S: - | 210000 | (38) |
| R2 | AACCATAGTGTCTCCACTAA |
Abbreviations: Bc, buffy coat; NPN, negative predictive number; PPN, positive predictive number; S, serum; Wb, whole blood.
aEncoding an immunogenic 31-kDa outer membrane protein, which is highly conserved with each known Brucella species and biovar (except B. ovis).
bEncoding a 26-kDa outer membrane protein of Brucella spp.
cEncoding a Brucella immunodominant antigen, named BP26, CP28, or Omp28 protein.
dUndetermined.
B4/B5 primers targeting bcsp31 are often used for the detection of eukaryotic brucellosis in clinical settings. This primer pair has the highest sensitivity (> 98%) in testing buffy coat or whole blood samples (40). Four primer pairs including B4/B5, JPF/JPR, P1/P2, 26A/26B, and F4/R2 can be applied in four distinct PCR assays to detect B. abortus, B. melitensis, B. suis, and B. canis at the genus level. These assays are ideal for rapid confirmation of human brucellosis (41). Two multiplex PCR assays, called AMOS and Bruce-ladder, are standardized and used to detect Brucella strains of animal or human origin (42). A multiplex PCR assay was described by Kumar et al. for the simultaneous detection of B. melitensis, B. abortus, and B. suis (43). Researchers reported various procedures that can detect and distinguish Brucella spp. in human serum and blood samples via a simple and robust multiplex PCR approach (44-46).
3.11. Other PCR-Based Approaches
Several nested and semi-nested PCR assays were developed to detect Brucella spp. in human blood samples (47). A nested-PCR assay was described for the diagnosis of relapse or chronic brucellosis in clinical practice (48). Both sensitivity and specificity was 100%. A semi-nested PCR assay for bcsp31 and IS6501 was evaluated on whole blood samples (49). However, these assays may increase the probability for primer-dimer formation and/or nonspecific amplification products. Moreover, the reported nested-PCRs can only detect a set of Brucella strains, but not single species. A novel loop-mediated isothermal amplification assay (LAMP) was developed to detect Brucella spp. DNA in human blood samples. The LAMP assay, based on the sequence of the highly repetitive omp25 gene, can detect 9 femtogram (fg)/µL of Brucella DNA with a sensitivity of 10 times higher than that of the nested-PCR (50).
Considering its advantages as simple operation, rapid amplification, and easy detection, the LAMP has potential applications for clinical diagnosis besides surveillance of human brucellosis in the developing countries without requiring sophisticated equipment or skilled personnel. An inexpensive and simple device such as a water bath or a heat block that can provide a constant temperature of 63°C is sufficient and, unlike conventional PCR result, it can be readout by the naked eye without the need for electrophoretic analysis (29). An arbitrarily primed-PCR (AP-PCR) to detect and identify 25 different Brucella strains is also introduced (51). Some PCR-restriction fragment length polymorphism (PCR-RFLP) techniques are successfully used to distinguish Brucella species and various biovars.
Restriction maps of omp2a and omp2b genes showed a greater diversity among and within Brucella spp. than other genes investigated so far. PCR-RFLP assays may serve as tools for diagnostic and epidemiological surveillance purposes (52). Furthermore, a PCR-enzyme immunoassay (EIA) was used by Vrioni for the diagnosis of human brucellosis directly from peripheral blood. Following the amplification of the bcsp31 target sequence, the amplified product was detected in a hybrid well-microtiter plate by hybridization analysis. The diagnostic specificity of the PCR-EIA for both whole blood and serum specimens was 100%, whereas the sensitivity was 81.5% for whole blood specimens and 79% for serum specimens. Vrioni et al. recommend that the detection of Brucella DNA in whole blood and serum specimens by PCR-EIA, as a sensitive and specific method, can help the rapid and accurate diagnosis of acute brucellosis (53)
3.12. Real-Time PCR Assay
The real-time PCR technique is more sensitive, specific, reproducible, and rapid than the conventional PCR. The quantitative real-time (qRT)-PCR allows both detection and quantification of the PCR product in real-time, while it is synthesized (54). Real-time PCR can be used for the rapid diagnosis of chronic, but serologically positive, brucellosis and acute brucellosis when serum and blood samples of known clinical presentations are investigated (40). These assays are developed targeting the 16S - 23S ITS region, IS711 element, and omp25, omp31, and bcsp31 genes (55-58). The bcsp31 gene target can be recommended for the detection of bacteria at the genus level. Species-specific identification confirming the primary diagnosis by a second gene target such as IS711 can be done (59, 60). Several multiplex real-time PCR approaches are developed for the simultaneous detection of Brucella spp. and Mycobacterium tuberculosis complex (MTC). These techniques amplify the bcsp31, IS711, and omp2a genes for the detection of Brucella spp. and target the senX3-regX3, IS6110, and cfp31 genes for the identification of the MTC (31, 32). Sanjuan-Jimenez et al. evaluated three molecular targets (IS711, bcsp31, and omp2a) of Brucella and three targets of MTC (IS6110, cfp31, and senX3-regX3) for their simultaneous detection by a multiplex real-time PCR (61).
3.13. Single Nucleotide Polymorphisms Typing
Some investigators previously described unique real-time PCR assays that can characterize Brucella isolates to the species level. They used single nucleotide polymorphisms (SNPs) multilocus sequencing (62). Foster et al. applied SNPs to housekeeping genes and introduced gene sequences that can identify the seven main Brucella species using the TaqMan assays with contained probes specific to each allele. The assays can detect DNA concentrations of less than 10 fg/mL that is their detection limit (63). However, finding SNPs that can separate B. canis from B. suis is challenging due to a high degree of sequence homology that indicates a recent split between these species (63).
3.14. Multilocus Variable Number of Tandem Repeats Analysis
PCR methods can detect Brucella spp. based on the finding of specific sequences, but limits of these techniques, for example, failure to differentiate among biovars within a species, encouraged the development of other molecular typing methods such as multilocus variable number of tandem repeats analysis (MLVA). Multilocus variable number of tandem repeats analysis measures the number of tandem repeats at a specified locus and can discriminate between isolates within a certain Brucella biovar. The MLVA is a quick and efficient method for typing and clustering Brucella strains. Moreover, multilocus sequence typing (MLST), sequencing of multiple genetic loci in bacteria, is increasingly accepted as a mean for the classification of microbial populations (64).
3.15. Matrix-Assisted Laser Desorption Ionization
The time of flight mass spectrometry (MALDI-TOF MS) is used as a fast and reliable technique for bacterial identification based on protein profile characteristics of microorganisms (65). MALDI-TOF MS is a reliable test for direct detection of Brucella to the genus level from blood culture bottles and culture plates. However, Brucella has not been yet incorporated into some of the main available databases due to its potential bioterrorism application (66). Another limitation of MALDI-TOF MS to detect Brucella is the need for pure cultures, which pose health hazards to laboratory personnel. Mesureur et al. described a simple and safe method for inactivation of Brucella isolates prior to their analysis by MALDI-TOF MS (67).
3.16. Novel Technologies for the Serologic Diagnosis of Brucellosis
The immunoblot-based assay showed several immunodominant proteins of B. abortus and B. melitensis in a previous study; this technique can be used to identify new candidate antigens for the serologic detection of brucellosis (68). Immunoproteomics of B. abortus RB51 (a mutant strain lacking the LPS portion) revealed several candidate antigens. The highly immunogenic proteins may be useful as alternative antigens to avoid cross-reactivity (69). Immunoproteomics of B. abortus also showed differential antibody profiles for B. abortus strain, S19-vaccinated and naturally infected cattle, and differentiation between vaccinated cattle and those animals infected with field strains (70).
4. Conclusions
Laboratory diagnosis of brucellosis still relies upon culture of bacteria followed by various biochemical and serological test results. Nucleic acid tests such as PCR are the novel-generation technologies that have higher sensitivity than blood cultures and better specificity than serologic tests. Molecular techniques such as PCR facilitate rapid, sensitive, and specific detection. MLVA is helpful for following an infection. Finally, it should be emphasized that novel technologies such as microfluidic lab-on-chip and next-generation sequencing (NGS) can provide a rapid, accurate, and safe diagnosis of brucellosis, especially in endemic countries. Future research on immunoproteomics and the selection of highly immunogenic protein spots can be useful as alternative antigens for the diagnosis of brucellosis.