1. Background
Hepatitis B virus (HBV) is one of the main causes of morbidity and mortality all around the world. It is estimated that one-third of the world’s population is infected with HBV and over 350 million people are chronic carriers. HBV infection results in 620,000 to one million deaths annually. As mentioned, 350 million people have chronic hepatitis B infection, which depends on age so that it is seen in 1% - 5% of adults and more than 90% of infected infants (1-3). The highest mortality rate caused by infectious disease is observed in children of less than five-years-old, especially in developing countries. Therefore, one of the main goals of the WHO is to reduce the mortality rate in children under five years. The prevention of hepatitis B infection is essential because of high prevalence, serious complications, and lack of definitive treatment (4, 5).
Vaccination is one of the most effective tools for the prevention of infectious disease in early life (6). In 1991, the WHO recommended that hepatitis B vaccine should be included in the National Immunization Program of Infants and Young Adults in all countries. The vaccination program, dose, and method of administration vary across countries. It depends on the formulation and the manufacturer’s instructions (7). The protective antibody titer can be usually evaluated three to six months after the last dose. A titer of above 10 mIU/mL indicates immunity to hepatitis B infection (8). The failure in the induction of the protective antibody is one of the main problems of vaccination and the persistence of the antibody level after primary vaccination is another problem (9). The efficacy of the antibody response to hepatitis B vaccine is estimated at 90% after the third dose in people under the age of 40 - 50 years (10). The duration of protection after vaccination is not exactly clear. However, many factors can affect the immune response to vaccines, such as:
- The quality of vaccine program: vaccine type, live vs. subunit vaccine, age at priming time, intervals between doses, vaccine potency, formulation and stability, vaccine administration, and scheduling.
- Host relative factors: sex, genetic factors, psychological stress, nutrition status, smoking, microbiota, breastfeeding, and maternal antibody.
- Environment factors: prenatal environment, perinatal environment, postnatal environment, and infections (11-14).
The birth season is one of the main environmental factors, which is associated with vaccination by the seasonal prevalence of pathogens, diseases, and other factors (15, 16). Toll-like receptors (TLRs) are the innate immunity receptors that have a role in the immune response to vaccines. Several studies indicated that TLR2, 3, and 4 play vital roles in the immune response to HBV infection by the secretion of pro-inflammatory cytokines and type Ι interferon (17-19).
2. Objectives
This study was designed to characterize the role of the birth season in some aspects of the immunological response to hepatitis B vaccine. We measured the HBV surface antibody (HBsAb) titers and the expression of TLR2, 3, and 4 genes in winter and summer-born children aged 3 to 5 years.
3. Methods
3.1. Study Groups
Two populations of healthy children, who were born in summer and winter, were enrolled in this study. Children in Group 1 were born in the summer season (14 males and 22 females; mean age of 4.05 ± 0.75 years). Children in Group 2 were born in the winter season (12 males and 24 females; mean age of 3.88 ± 0.85 years). Children in both groups were born between March 2011 and March 2014 and were vaccinated with three doses of recombinant hepatitis B vaccine at birth and one and six months after birth according to the vaccination program. The inclusion criteria were children born in winter or summer between March 2011 and March 2014. The written informed consent was taken from each patient and parents of children. The exclusion criteria were chronic diseases possibly affecting vaccination, such as infections, thalassemia, diabetes, SLE, and other systemic diseases. Four milliliters of blood samples in EDTA were collected from each subject. The season of birth was defined as summer (June to September) and winter (December to March).
3.2. Isolation of Peripheral Blood Mononuclear Cells
Three milliliters of fresh whole blood were obtained from each subject. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll Histopaque density gradient (Biosera, England). PBMCs were washed three times with Phosphate-Buffered Saline (PBS) and pellets were suspended in an RNX-plus solution (SinaClon, Iran).
3.3. RNA Extraction from Blood Samples and Real-Time PCR
Total RNA was extracted by the RNX-plus Kit (SinaClon, Iran) according to the modified manufacturer’s protocol. The purity of total RNA was evaluated using a NanoDrop spectrophotometer to determine the quantity, protein contamination, and organic solvent contamination. For reverse transcription (RT), 0.1 ng to 5 µg of total RNA were transcribed using the two-step cDNA Synthesis Kit (YektaTajhiz, YTA, Iran) following the manufacturer’s instructions. Real-time PCR was performed using 1 µL of DNA template and 2x SYBR Green qPCR Mix (YektaTajhiz, YTA, and Iran).
The gene-specific primers were selected as described in Table 1. Quantitative real-time PCR started at 95°C, followed by subsequent amplifications including denaturation for 10 seconds at 95°C, annealing (for 20 seconds at 58°C for TLR2 and 59°C for TLR3, TLR4, and GAPDH), and extension for 30 seconds at 72°C. Overall, 35 cycles were used for TLRs. The PCR products specificities were measured using a melting curve and 1.8% agarose gel. The relative expression of genes was quantified by 2-ΔΔCT and the results were normalized by the GAPDH gene.
| Target Gene | Sequence (5’ - 3’) | Accession Number | |
|---|---|---|---|
| Forward | Reverse | ||
| TLR-2 | AACTGGTAGTTGTGGGTTGAAG | CGGTCACAAGACAGAGAAGC | NM-001318789.1 |
| TLR-3 | TCTGTCTCATAATGGCTTGTCA | AACACCCTGGAGAAAACTCTT | NM-003265.2 |
| TLR-4 | CCGTTTTATCACGGAGGTGG | CAGGTCCAGGTTCTTGGTTG | NM-003266.3 |
| GAPDH | GGTGGTCTCCTCTGACTTCAACA | GTTGCTGTAGCCAAATTCGTTGT | NM-001289746 |
Primer Sequences Used to Detect TLRs in Real-Time PCR
3.4. Hepatitis B Surface Ab Test
The serum samples were collected from each subject and stored at -80 for enzyme-linked immunosorbent assay (ELISA) (PishtazTeb, Iran). Antibody titer of > 10 mIU/mL and antibody titer of ≤ 10 mIU/mL represented the responder and non-responder groups, respectively.
3.5. Statistical Analysis
Data were reported as the mean and standard error of the mean. Differences between the two groups were determined by the t-test and P ≤ 0.05 was considered statistically significant.
4. Results
4.1. Demographic and HBs Ab Titer in Subjects
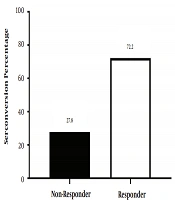
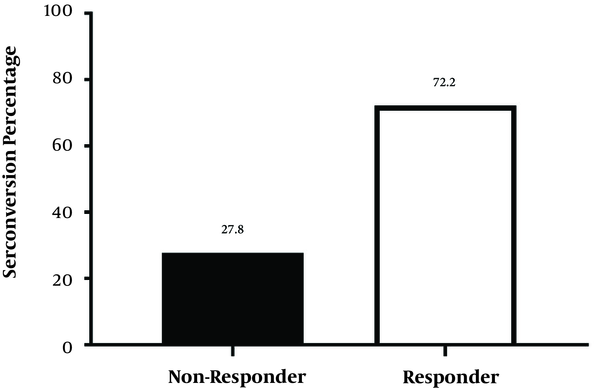
There were 26 girls (36.2%) and 46 boys (63.8%) among subjects. Among them, 52 subjects (72.2%) had HBs Ab titer more than 10 mIU/mL and were responders, while 20 subjects (27.8%) had HBs Ab titer less than 10 mIU/mL and were non-responders (Figure 1). Thirty-three subjects (71.7%) among boys were responders, and 13 of them (28.3%) were non-responders. Nineteen subjects (73%) among girls were responders, and seven of them (27%) were non-responders. There was a significant relationship between gender and HBs Ab titer (P value = 0.04).
4.2. The Association Between Birth Season, TLR 2, 3, and 4 Expressions, and HBsAb Titer
Table 2 shows the mean ± SEM of the relative expression of TLR 2, 3, and 4 genes and HBsAb titer in the summer-born and winter-born groups. The expression of TLRs genes was higher in the winter group than in the summer group, but no statistically significant difference was detected. The HBsAb titer was higher in summer-born children than in winter-born children (48.46 ± 10.03 vs. 37.52 ± 8.5, P value = 0.3). We investigated the HBsAb titer in the responder and non-responder groups according to birth season and the results are shown in Figure 2. There were no differences in HBsAb between summer-born and winter-born children among responders and non-responders. Moreover, there was no relationship between Ab titer and the expression of TLRs.
| Variables | Summer (N = 36) | Winter (N = 36) | P Value |
|---|---|---|---|
| TLR2, ratio | 0.1073 ± 0.22 | 0.1322 ± 0.02 | 0.5 |
| TLR3, ratio | 0.0738 ± 0.01 | 0.1032 ± 0.02 | 0.06 |
| TLR4, ratio | 0.0635 ± 0.01 | 0.0795 ± 0.01 | 0.16 |
| HBs Ab titer, mIU/mL | 48.46 ± 10.03 | 37.52 ± 8.5 | 0.3 |
The Comparison of Relative Expression of TLRs Genes Between Summer and Winter-born Children Aged 3 - 5 Yearsa
4.3. Comparison of TLR 2, 3, and 4 Expressions in Responder and Non-Responder Groups
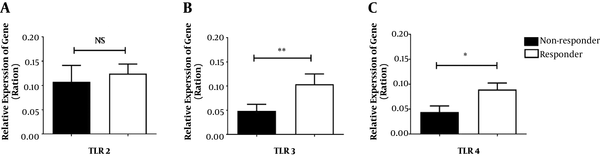
We compared the relative expression of TLR 2, 3, and 4 genes between responder and non-responder subjects (Figure 3). The mean ± SEM of the relative expression of TLR 3 and TLR 4 genes was higher in the responder group than in the non-responder group (TLR-2: 0.1244 ± 0.019 vs. 0.1075 ± 0.033, P value = 0.66) (TLR-3: 0.1039 ± 0.021 vs. 0.04873 ± 0.013, P value ≤ 0.001) (TLR-4: 0.08932 ± 0.013 vs. 0.04414 ± 0.012, P value = 0.0.012). There was a significant association between the expression of TLR 3 and TLR 4 and the efficacy of the hepatitis B vaccine, but this association was not significant for TLR 2.
5. Discussion
The current study examined the expression level of TLR 2, 3, and 4 and the HBsAb titer in PBMCs and serum samples of children born in winter and summer. This study demonstrated that the birth season could not affect the expression levels of TLR 2, 3, and 4. We showed that the relative expression of TLR 2, 3, and 4 was higher in the winter group than in the summer group; however, this difference was not statistically significant (P value ≥ 0.05). Several studies showed that the birth season modulated the immune system function (15, 16, 20, 21). Birth season considerably influences innate immune function in early life. Winter-born infants indicated decreased TLR3-mediated IL-12p70 and increased TLR7-mediated IL-10 that might be associated with the augmentation of Th2-polarization and predisposition to allergy and asthma (22). The concentrations of monocytes and pDC were higher in winter months than in summer months in neonates (20).
Interestingly, a study demonstrated that leukocyte counts, type 17-related immune mediators, and IL-5 were more in winter newborns than in summer newborns (15). Dopico et al. showed that the immune cell composition varied by season and it might affect the immune system. This study also reported that the expression of the co-regulated pro-inflammatory gene, total monocyte numbers, the concentration of sIL-6R, and CRP in blood samples increased in winter and these increases promoted inflammation and were associated with an increased incidence of rheumatoid arthritis and type 1 diabetes during winter months (23). Indeed, the expression of TLR7, TLR8, and DDX58 (encoding the viral RNA receptor RIG-I) increased in winter months, which predicted the protective immune response to the Yellow Fever vaccine (24). Generally, these studies suggested that immune cell numbers and immune responses could change by season and exposure to seasonal pathogens, which may trigger inflammation or prime the immune system. We also found that the mean value of HBsAb titer was higher in summer-born children than in the winter group, but this relationship was not statistically significant (P value ≥ 0.05).
Several studies supported our findings. Studies showed that daylight, the human circadian clock, and ambient temperature were the three main factors that could modulate the immune system (23, 25). The circadian rhythm could also regulate inflammatory innate immune function (26). Moreover, the circadian system regulated the immune cell counts and functions and the release of hormones was mediated by the circadian clock (25). Melatonin is another factor that is related to the circadian clock and its peak level occurs at night. Melatonin can modulate the immune system; for example, it regulates monocyte (CD14+/CD4+ cells), neutrophil, and T cell activities and increases NK cell activity, ADCC activity, production of GM-CFU, and the synthesis of both thymosin a1 and thymulin. IL-2 production can be elevated by melatonin, which shifts T cell differentiation toward the Th1 phenotype and enhances IFN-γ generation (27).
The sunlight elevates the production of reactive oxygen species (ROS) and some mediators such as prostaglandins, histamine, IL-4, IL-1β, and TNF-α, promotes mast cell, DC, NK-T, T-reg, and macrophage functions, and decreases IL-12 and Th-1 cytokine production (28). Although sunlight can be immunosuppressive, it is beneficial for vitamin D synthesis. The sunlight is necessary for vitamin D synthesis in the skin. Andersen et al. reported that the vitamin D level was lower in the winter season than in the summer season among adolescent girls and elderly women (29). On the other hand, the clothing style is one of the crucial factors that can affect vitamin D absorption.
In the winter season, the wearing of heavy clothing and the limited exposure of the skin surface to sunlight have significant influences on vitamin D concentration that may result in vitamin D deficiency (30). Concerning the role of vitamin D, there was a positive correlation between vitamin D concentration and hepatitis B vaccine efficacy. The probable hypothesis for this observation concerns the role of vitamin D in DC migration and induction of adaptive immune responses (31). Some studies demonstrated that vitamin D augments IL-10 production and drives T-cell differentiation to Th-2. In addition, the expression of TLR 2 and 4 was suppressed by vitamin D (32). These findings explain the increased HBsAb titer in summer-born children in our study.
Overall, many seasonal factors, including seasonal allergens, pathogens, vitamin D, and sunlight, might be responsible for the response to vaccines (15, 23, 31, 32). The seasonal pattern prevalence of pathogens has a conflicting role in immune responses to vaccines. They can act as adjuvants and boost Ab responses or interfere with the induction of protective Ab and suppress immune responses to vaccines (33). Future studies are needed to evaluate the roles of these factors in the immune system function.
Our study also compared the expression of TLRs in responder and non-responder groups. The expression of TLR 2, 3, and 4 was higher in the responder group than in the non-responder group. Zhang and Lu reported that TLR 2, 3, and 4 had some effects on pro-inflammatory cytokines and the secretion of interferons, which inhibited HBV replication (17). The expression of TLR 2, 3, and 4 in PBMCs of chronic hepatitis B patients can be down-regulated by HBsAg, and it was related to established chronic infection (34). Accordingly, TLR 2, 3, and 4 have some effects on the induction of protective Ab against hepatitis B infection, which confirm our results.
We investigated the HBsAb titer in our study. We showed that 72.2% of the subjects were responders and 27.8% were non-responders. The results of several studies were similar to our results. The efficacy of HBV vaccination was 82.82% in Iran (35), 90.9% in the city of Tehran (36), and 88% in India (37). Observations have shown that the HBsAb level differs according to sex. In our study, boys had a better response to hepatitis B vaccine than girls. However, there are controversial results in this regard. Although some studies indicated that girls had a better response to hepatitis B vaccines (12, 35), Ferraz et al. showed that males had better responses than females (38). Moreover, some other studies indicated no association between gender and immunological response to HBV vaccine (1, 39). Therefore, the mechanisms are still unknown, but it is not related to sex hormones.
This study had several limitations. First, the sample size was small. Second, the current study investigated two seasons (summer and winter) as birth seasons in children. Third, it did not measure the concentrations of protective Ab in mothers. It is proposed to investigate the expression of other innate immune receptors such as TLR 1,6,7, RIG-I, interferon Type I and II, and vitamin D level, which have roles in hepatitis B immunology.
5.1. Conclusions
This study demonstrated that multiple environmental factors such as birth season might be associated with children’s immunological responses to hepatitis B vaccine. The birth season could influence immune responses to vaccines due to variations in the pathogenic pattern, daylight, and weather; these factors may induce or suppress the subsequent response to the vaccine.