1. Background
Nanoparticles (NPs) are small-sized particles with less than 100 nm diameter that exhibit large surface area to volume ratio. To have a small size is taken account of an advantage that the nanoparticles have significant applications in the medical field (1). Microbial infections have been known to cause various diseases worldwide. Though the field of medicine was revolutionized by the discovery of antibiotics (2). However, over time, the increasing appearance of antibiotic resistance has reduced their efficacy against various infections, which has resulted in the emergence of more and more strong pathogens (3) and raised the need for the development of new and efficient antimicrobial agents against such resistant pathogens (4).
The use of nanoparticles is presently gaining more attention to their chemical, optical and mechanical properties. Nanoparticle-based drug delivery is an emerging concept in therapeutics and diagnostics issues from the last two decades (5). Due to the small size, NPs can easily penetrate bacterial cells, which are coming up as the current interest in the researchers to be used as an attractive and alternative therapeutic approach against drug resistance. Moreover, they are found to be the most promising therapeutic tools as they show high-quality antibacterial properties (6). Among various types of metal nanoparticles, those synthesized from silver metal (silver nanoparticles, AgNPs) have received more attention in the biomedicine. The AgNP’s have been found to show low toxicity profiles along with antiseptic and antimicrobial activity against Gram-positive and Gram-negative bacteria. Therefore, the green biosynthesis of AgNPs is an attractive field to work for the researchers to find a robust solution for the emerging antibiotic resistance problem (7).
Besides physical and chemical procedures on which a lot of studies have been performed, the biological synthesis of AgNPs has received more considerable attention as it is found to be human friendly based on the green chemistry principles. Relying on the utilization of universally accepted solvents, harmless capping, and stabilizing agents, this biological synthesis approach has been found to increase the biocompatibility of the resultant products with healthy tissues for in vivo applications. There are a few microbes that have a unique ability of resistance against metal ions. Bacteria are found to possess an outstanding ability to reduce heavy metal ions and are considered as one of the best biological sources for nanoparticles synthesis (8).
Microbial synthesis of silver nanoparticles can be achieved by adopting either an intracellular or extracellular way of synthesis. Extracellular synthesis of nanoparticles is preferred over intracellular synthesis due to the ease of the procedure. It is found economical and can be achieved through simple downstream processing that does not require additional steps for releasing and collection of nanoparticles from biomass. Previous studies have found that the microbial enzymes are found to play a vital role in achieving extracellular bio-reduction that involves the efficient transport of electrons. since the past studies, it has been revealed that extracellular synthesis of silver nanoparticles is due to nitrate reductase enzyme (1), which causes the efficient conversion of nitrate into nitrite transferring an electron to the silver ion (Ag+ to Ag0) (9). Besides, this reducing non-enzymatic mechanism has also been found to be a way of AgNPs synthesis it can involve in the bio-reduction by organic functional groups present in the microbial cell walls. The present study focuses on the efficient synthesis of AgNPs through a bio-friendly strain Bacillus subtilis.
2. Objectives
The aim was to synthesize AgNPs by using a specific strain of B. subtilis on which previously no work has been done to check its antimicrobial activity against MDR bacteria. This work was done to find a potent antimicrobial agent against those MDR strains, which has been recently mentioned by WHO as a serious threat to mankind.
3. Methods
3.1. Source of Microorganism
The bacterial test strain Bacillus subtilis (FCBP-WB-0174) was obtained from the Fungal Culture Bank of Pakistan (FCBP), Punjab University, Lahore. The obtained pure culture was maintained on LB agar (Sigma Aldrich, Germany) and sub-cultured to regulate its viability in the microbiology laboratory (Department of Microbiology, University of Central Punjab, Lahore, Pakistan) over a period of time.
3.2. Metal Resistivity Test
Silver nitrate was used as a source of Silver metal against which resistance was tested. Testing of silver metal resistivity of all B. subtilis strains was performed using the methodology which was previously described (10). The colonies of all four strains (FCBP-SB-324, FCBP-SB-223, FCBP-WB-0174 and FCBP-SB-0189) were allowed to grow on AgNO3 supplemented nutrient agar. Filter sterilized 1 M AgNO3 solution was added into the prepared medium before solidification and then allowed to cool. On solidification, strains were allowed to grow on supplemented agar. The strain showing growth on the supplemented agar was selected for Nanoparticles synthesis.
3.3. Nanoparticles Synthesis Protocol
Nanoparticles derived from the culture supernatant of B. subtilis were synthesized using the methodology according to a previous study with some modifications (11).
3.4. Production of Biomass
The bacterial strain was cultured in nutrient broth medium to produce biomass. Three flasks containing 100 mL media broth each were inoculated with 1 µL of bacterial strain culture broth. All flasks containing inoculated broth were placed in a shaking incubator at 37°C and under the shaking condition of 150 rpm for 24, 48, and 72 hours, time dependently. After 24 hours of bacterial growth, the liquid broth containing grown culture was centrifuged for 20 minutes at 8000 rpm for the separation of bacterial culture and supernatant. After centrifugation, the clear culture supernatant was collected in a sterile flask. The same procedure was also repeated at 48 and 72 hours through the previous manner for collecting culture supernatant.
3.5. Extracellular Biosynthesis of Silver Nanoparticles Using Culture Supernatant
For the biological synthesis of silver nanoparticles, 1 mM sterile silver nitrate solution was treated with the bacterial supernatant solution in a ratio 1:1 in a 250 mL Flask. The reaction mixture was placed under bright conditions in shaking incubator, at 37°C for different time points (24, 48, and 72 hours) at 150 rpm. After 24, 48, and 72 hours, the reduction of the silver ions to silver atoms was observed in a time-dependent manner. Control experiments were also conducted by placing silver nitrate solution and media as positive and negative controls under the same conditions in shaking incubator to check for the bacteria role in the nanoparticles synthesis. Control samples without culture supernatant were placed under the same conditions as the reaction mixture containing the supernatant. The extracellular synthesis of AgNPs was monitored by observing the color change from pale yellow to deep brown. The appearance of brown color is the first indication of the synthesis of AgNPs that can be a result of the reduction of silver ions to the silver atom.
3.6. Characterization of AgNPs
The reduction of the Ag+ ions was observed by obtaining UV-VIS absorbance spectra on a HALO-DB, UV-VIS Double Beam Spectrophotometer with samples in glass cuvettes. For analysis of Ag+ ions reduction, 2 mL aliquot of the sample from the bulk material was discarded at intervals of 24 hours, until a dark brown color was observed. The absorbance spectra were measured and recorded in the range of 300 - 800 nm with a wavelength step size of 10 nm at room temperature (RT). Size and surface morphology analysis of the extracellular synthesized silver nanoparticles was performed by scanning electron microscopy (SEM) (Zeiss EVO LS10 VP-SEM, Germany). Samples for SEM analysis were prepared by drop coating the colloidal AgNPs solution on a glass slide and were allowed to dry. The dried sample was loaded onto a specimen holder and observed under the magnifying glass. The size distribution profile of silver nanoparticles was analyzed and studied by using Malvern Zeta sizer version 7.11. The size analysis was done by using glass cuvettes with a round aperture and water as a dispersant medium. The measurements were taken at 30°C for 24 seconds.
3.7. Antibacterial Activity
The synthesized and well-characterized silver nanoparticles were checked for their antimicrobial activity against four pathogenic bacterial strains obtained from a local hospital in Lahore including Acinetobacter baumannii, Pseudomonas aeruginosa, Methicillin-Resistant Staphylococcus aureus (MRSA) and Escherichia coli. Antimicrobial activity of AgNPs against all test pathogenic strains was assessed by Disk Diffusion Method. These pathogenic bacterial strains were isolated by following the protocols of various previous studies (12-14). McFarland standard was prepared for each bacterial strain. A sterile swab was dipped into the broth culture of the test organism and streaked over the MHA (Muller Hinton Agar) plate to form a lawn of growth. Under sterilized conditions, 6mm wicks paper discs were placed on the plates containing growth media. About 100 μL of AgNPs suspension was impregnated in each disc and gently pressed by sterilized forceps. Two other disks with 100 μL of AgNO3 solution and culture supernatant were placed as a positive and negative control on each plate. The Petri-plates were labelled carefully and incubated at 37°C for 24 hours to allow the bacteria to grow and to check the AgNPs activity. After 24 hours, the antibacterial activity was measured by measuring the clear inhibition zones in mm scale using zone reader.
3.8. Statistical Analysis
All the experiments were performed in triplicates and data was analyzed using SPSS and GraphPad Prism 7. The results were described as mean and standard deviation if it is applicable (SD).
4. Results
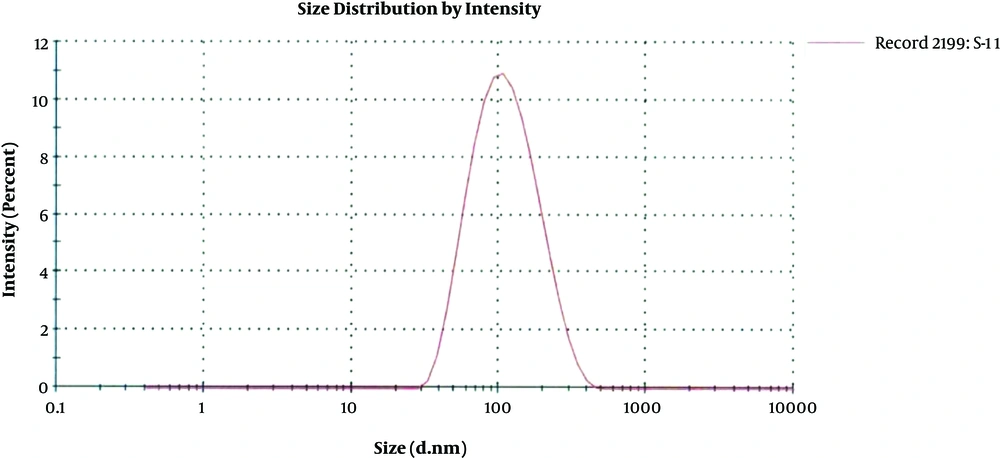
Among all the four B. subtilis strains obtained from FCBP, only FCBP-WB-0174 was found to be metal (silver) resistant whose culture supernatant was later on used for nanoparticles synthesis. The size distribution graph of the colloid silver nanoparticles using different concentrations of silver nitrate and bacterial culture supernatant achieved various populations of nanoparticles. The mean size obtained from the AgNPs synthesized from 1 mM AgNO3 concentration yielded only one population of nanoparticles with an average diameter of about 89.49 nm supporting the results obtained from the SEM analysis. Remaining two colloidal solutions caused the multiple populations of AgNPs. In addition, particles synthesized from 2 mM AgNO3 solution gave two different peaks: 76.84 nm diameter with 97.5% intensity and 5030 nm diameter with 2.5% intensity. That also supports the results obtained from SEM second concentration 84 nm and the second peak.
The remaining colloidal particles gave three peaks with diameter 83.81, 10.14, and 4785 nm with 79.6, 18.0, and 2.4% intensities. These results also supported the SEM analysis of the remaining two colloidal solutions that yielded particles with both small and large sizes. However, among all the samples, the nanoparticles synthesized from 1 mM AgNO3 solution contained the highest concentration of good quality nanoparticles. The change in color of the reaction mixture were observed by adding AgNO3 and different types of culture supernatants (Figure 1). A dark brown color was observed after 72 hours of reaction mixture formation. Several culture supernatants to AgNO3 ratios (1:1 to 1:6) were tested to optimize the production of AgNP. From culture supernatant of 24 and 72 hours grown B. subtilis, there was a slight change in color even after 72 hours of reaction in nutrient broth. From culture supernatant of 48 hours grown B. subtilis in nutrient broth, there was a clear change in color when mixed with AgNO3 in 1:1 ratio, under 37°C and shaking condition of 150 rpm. However, the change in color was found to decrease with the increasing concentration of AgNO3 in the culture supernatant, which indicated the synthesis of silver nanoparticles from 48 hours of old culture supernatant.
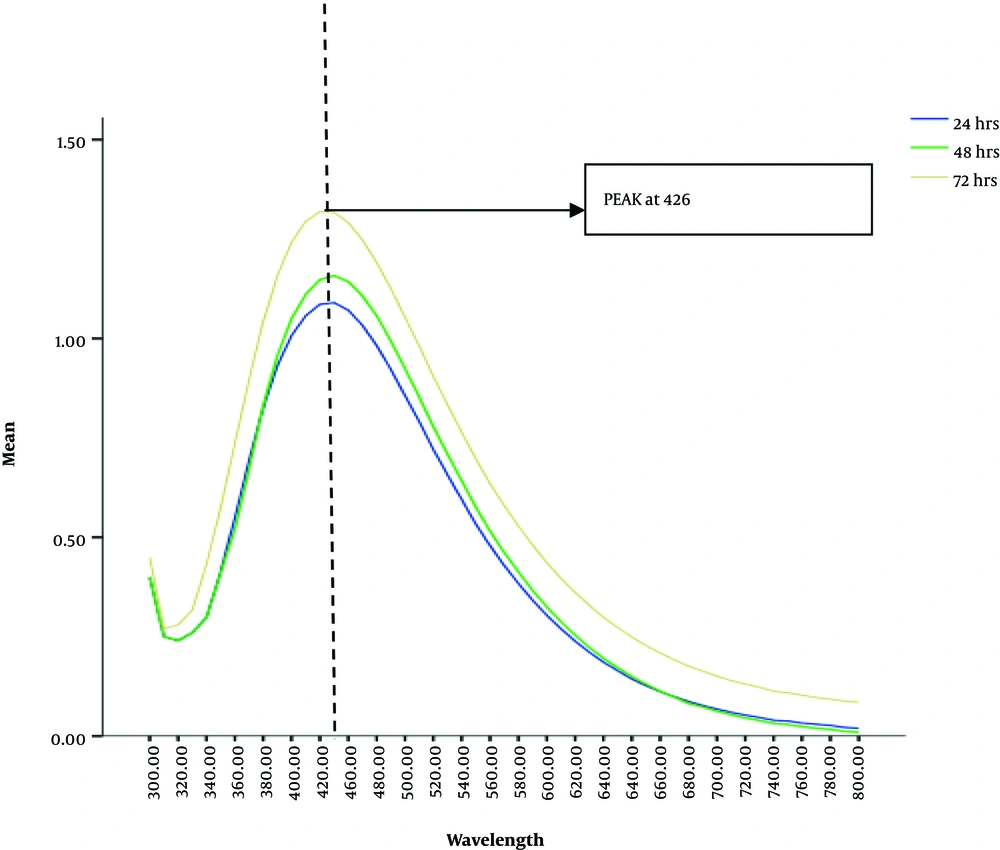
For initial confirmation of the AgNPs synthesis, UV-Vis spectra of the solutions that gave characteristic brown color, was obtained. Sharp peaks at 426 nm wavelengths were observed by all the aliquots of solution mixture taken after 24, 48 and 72 hours. However, the sharpest peak was observed in the old reaction mixture from 72 hours (Figure 2). Scanning Electron Microscopic micrographs were obtained at almost 50,000 to 75,000 magnification. The micrographs were recorded from a drop coated film of an aqueous suspension of biologically synthesized AgNPs. These micrographs indicated a characteristic size for ideal nanoparticles with an average diameter of about 80 nm, which was less than 100 nm (Figure 3).
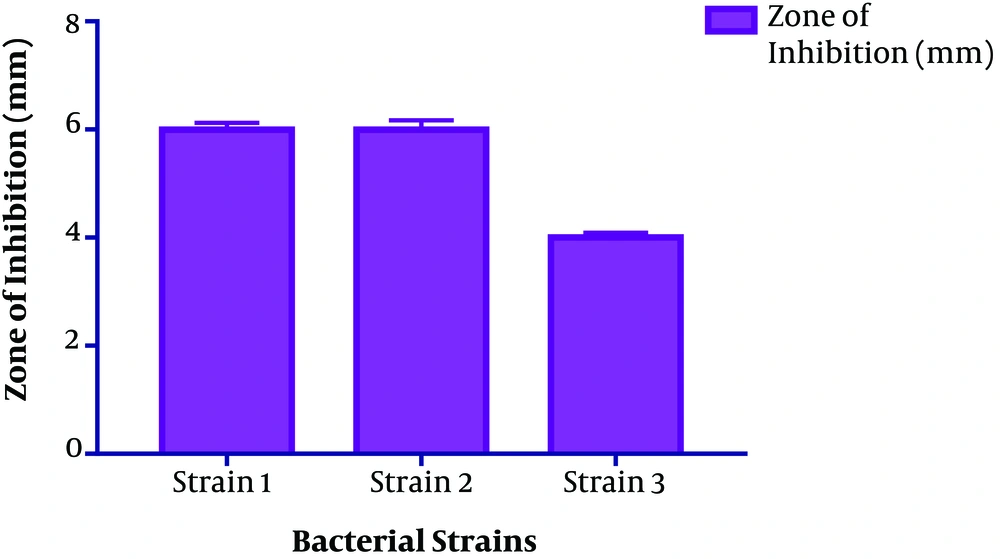
The size distribution analysis of colloidal nanoparticles was obtained from the AgNPs colloidal solution synthesized from 1 mM silver nitrate solution and culture supernatant provides only one population of nanoparticles with an average diameter of about 89.49 nm. (Figure 4). The antimicrobial activity of the microbial synthesized AgNPs was assessed against four multidrug-resistant strains A. baumannii, P. aeruginosa, Methicillin-Resistant S. aureus (MRSA) and E. coli. Vigorous antibacterial activity was shown against A. baumannii and P. aeruginosa as well as an acceptable effect on MRSA (Table 1 and Figure 5).
| Serial No. | Target MDR Bacterial Strains | Zone of Inhibition (mm) |
|---|---|---|
| 1 | A. baumannii | 06 ± 0.12 |
| 2 | P. aeruginosa | 05 ± 0.17 |
| 3 | MRSA | 04 ± 0.09 |
| 4 | Escherichia coli | 06 ± 0.12 |
5. Discussion
The appearance of brown color was an initial indication of the silver nanoparticles synthesis in the reaction mixture using bacterial culture supernatant. The characteristics brown color of the colloidal solution is reported to be due to the surface plasmon vibrations in nanoparticle and provides a spectroscopic indication of their formation (15). The change in color is found due to the reduction of silver ions into silver atoms in the presence of aqueous enzymatic extract from the culture supernatant. Studies have shown enzymes and reaction substrate to be responsible for the formation of silver atoms showing their characteristic brown color.
From culture supernatant of 48 hours grown B. subtilis in nutrient broth, there was a substantial change in color; however, from the culture supernatant of 24 and 72 hours grown B. subtilis, there was no noticeable deep brown color change even after 72 hours of reaction at any concentration. This indicated the absence of the synthesis of silver nanoparticles (16, 17). This can be supported by the fact that the OD of the bacterial growth was recorded at the highest level after 48 hours old culture which may indicate the maximum amount of enzyme concentrations secreted by the bacterial culture in the broth (18). 24 hours old culture may contain less enzyme concentrations due to the low number of bacterial colonies and 72 hours old culture broth may also contain less bacterial growth due to the decline phase of the bacterial life cycle. Therefore, we reported maximum enzyme production in 48 hours old grown culture broth giving the maximum deep brown characteristic color of AgNPs (19). Under UV-VIS spectrophotometric scanning, all the aliquots of the reaction mixture obtained from 48 hours old culture supernatant gave sharp peaks at 426 nm (19).
These results indicated that the presence of AgNPs, is a characteristic wavelength range for silver nanoparticles. The peak value also revealed the absorption value of the particles in solution, which can also be explained by the surface plasmon resonance (SPR). The excitation of the surface plasmon vibration is the base of change in color to brown. Similar observations have also been reported in other researches (20). Scanning electron microscopy analysis confirmed the average particle size of about 80 nm diameter and also do not show aggregation in AgNPs synthesized from 1 mM AgNO3 concentration and culture supernatant in 1:1 ratio. The shape of the nanoparticles was found spherical in the SEM micrographs as reported by many other kinds of research on microbially synthesized nanoparticles. The size and shape could be closer to other researchers reported on the bacterial synthesis of AgNPs. Khan et al., have also reported the formation of circular-shaped AgNPs within a range of 50 - 100 nm in size that supported our research (21).
The size distribution analysis supported the results obtained from the SEM analysis as well as confirmed the presence of silver nanoparticles as the average size of nanoparticles obtained within a range of characteristic nanoparticles. The antibacterial activities of nanoparticles have been proved through different studies to be dependent on their size, shape and stability. Their antimicrobial activity has been reported to be inversely related to size and shape (22, 23). The higher surface to volume ratio of silver nanoparticles increases their contact with microorganisms, which promotes the dissolution of silver ions hence improving the bactericidal effectiveness of nanoparticles (24). Moreover, studies also show that despite using different microbial species for nanoparticles synthesis, the results may vary in size and shape.
This observation can also be found even using the same species but varying different parameters of the process, like pH of the precursors, incubation time, precursor concentration, etc. Mathew et al. in their studies have shown, the antimicrobial activity of the biosynthesized silver nanoparticles to be size and shape dependent (25). The previous studies showed that the presence of natural stabilizing and capping agents in biosynthesized AgNPs make them more antibacterial than chemically synthesized AgNPs (26). The antimicrobial activity of AgNPs has been reported against both Gram-positive and Gram-negative bacterial strains (15, 19). In our study, antimicrobial assessment of microbial-induced AgNPs synthesis from B. subtilis gave positive results against all four strains. Strong antibacterial activity was also shown against A. baumannii (06 ± 0.17) and P. aeruginosa (05 ± 0.17) and moderate results were shown on MRSA strain (04 ± 0.09).
The results of our study proved the microbial-induced silver nanoparticles synthesis from Bacillus strain as a potential antimicrobial agent. The antibacterial activities of nanoparticles have been proved through different studies to be inversely dependent on their size, shape and stability. The higher surface to volume ratio of silver nanoparticles increases their contact with microorganisms. This promotes the dissolution of silver ions hence improving the bactericidal effectiveness of nanoparticles (24). Moreover, the antimicrobial potential of AgNPs against bacteria has been found to exist through various mechanisms.
AgNPs can adhere to the surface of microbial cell wall and membrane, and then their penetration into the cell is one of the modes of action of AgNPs. On the other hand, they cause damage to the bimolecular components such as protein, lipids, and DNA as well as intracellular structures such as mitochondria, vacuoles, ribosomes inside the cell. Besides this, they have also been found to generate reactive oxygen species (ROS) and free radicals to induce cellular toxicity and oxidative stress leading towards cell damage (23). Control of microbial signal transduction pathways has also been recognized as one of the prominent modes of antimicrobial action. Results of the present study indicated that the microbial-induced silver nanoparticles synthesis are potent antimicrobial agent against strong infectious microbial agents and can be used in the future against multidrug-resistant strains.
5.1. Conclusions
Bacillus subtilis strain used in our study, demonstrated the ability to synthesize silver nanoparticles extracellularly by bio-reduction from 1 mM AgNO3 precursor solution. The microbial-induced silver nanoparticles synthesis from Bacillus subtilis have the potential to act against various resistant pathogenic strains. Their synthesis was found best from nutrient media, at 1:1 concentration of culture supernatant and AgNO3 after 72 hours of incubation. The spherical AgNPs with an average diameter of 80 nm was found through SEM analysis, and an ideal peak at 426 nm was found through UV-analysis. Size distribution analysis further confirmed the single population of the particle with a size around 80 nm that confirmed the presence of silver nanoparticles. These particles were found effective against various pathogenic drug-resistant strains. This finding indicated their potential antimicrobial activity against various infections.