1. Background
Pseudomonas aeruginosa is a Gram-negative bacillus. It is a ubiquitous bacterium that can be found in a wide range of environments. The National Nosocomial Infection Surveillance System has declared P. aeruginosa as a leading cause of nosocomial infections, including septicemia, cystic fibrosis, burn wound, respiratory and urinary infections (1-3). Pseudomonas aeruginosa infections have become a real concern since many of the available antibiotics appear to be poorly effective against this bacterium. Besides, the excessive use of antibiotics during treatment accelerates the ineffectiveness of empirical antibiotic therapy against P. aeruginosa (4). Hence, the Center for Disease Control and Prevention has placed P. aeruginosa among the top 10 resistant bacteria worldwide.
The extraordinary resistance and pathogenicity of P. aeruginosa are principally due to the function of quorum sensing (5). Quorum Sensing (QS), a cell-cell communication system, plays a critical role in survival and colonization and is responsible for controlling more than 10% of P. aeruginosa genes. These genes are mainly involved in antibiotic resistance, motility, production of virulence factors, and biofilm formation (6). Among the QS genes, the dominant regulator is called the las system, which utilizes Acylated Homoserine Lactones (AHLs) for signaling. The synthase of las QS is termed LasI, which stimulates receptor LasR (1). LasR homodimerizes after binding their signal molecules and prompting their transcription. On the other hand, PqsR (known as MvfR) is a transcriptional regulator that links to the promoter area of the pqsABCDE operon and instantly regulates its expression to produce an auto-regulatory loop (7). Apart from its intrinsic resistance, P. aeruginosa possesses an outstanding ability to develop resistance through the selection of mutations in a complex network of genes implicated in resistance and gene regulation (8). Noticeably, mutations can affect protein expression, subcellular localization, protein folding and stability, and protein function (9). An increasing pool of evidence suggests that the amino acid sequence of a protein determines its final structure, which, in turn, determines its function. Protein destabilization or some abnormal biological functions would be the results of these mutations. While the majority of non-synonymous Single-nucleotide Polymorphisms (nsSNPs) seem to be functionally neutral, the others show functional consequences and may cause or influence diseases. When engineering proteins, it is vital to know the extent of mutation impact on the stability of new proteins in contrast to the wild-type (10). The increasing trend in antibiotic resistance has caught attention to the improvement of new treatment strategies, such as reducing bacterial virulence through QS (11).
Since the QS system plays a crucial role in regulating P. aeruginosa virulence, it is regarded as a promising drug target. Seemingly, the suppression of the QS system converts pathogenic P. aeruginosa into nonpathogenic without using standard antibiotics; it is a new strategy of antibacterial therapy (12). That is why identifying and reporting new variants that are likely to be pathogenic are significant to study.
2. Objectives
This study aimed to examine the possible mutations in lasR and pqsR genes in extremely drug resistant (XDR) and multidrug resistant (MDR) strains of P. aeruginosa from burn wound infection.
3. Methods
3.1. Identification of Clinical Isolates
In this study, 120 samples belonging to burn patients from Imam Musa Kazem burn injury hospital were sent to the microbiology laboratory of the Isfahan University Medical Sciences, Isfahan, for identification and other processes between January and June 2015. The clinical isolates were plated on blood agar (Merck, Germany) and Cetrimide agar (Merck, Germany). Then, the plates were incubated at 42ºC aerobically for 24 hours. The bacteria shape and colony morphology were assessed. We also conducted standard microbiological tests, including catalase, oxidase, triple sugar iron agar, indole production, and oxidative-fermentative tests (Merck, Germany) (Figure 1).
3.2. Antibiotic Susceptibility Testing
We chose 10 types of antibiotics (Mast, England; Padtan Teb, Iran) to cover a wide range of antibiotic activity for assessing the bacterium behavior. The antibiotics are listed in Table 1. Antibiotic resistance patterns of P. aeruginosa isolates were determined by the Kirby-Bauer disc diffusion method according to the Clinical Laboratory Standard Institute (CLSI) guidelines, version 2015. As a reference strain, P. aeruginosa ATCC 27853 was used in the present study. First, a microbial suspension (equivalent to 0.5 MacFarland) was prepared directly from standard strain ATCC 27853 and cultured on Mueller Hinton agar (Merck, Germany) for the quality control of antibiotics (13). Finally, discs were placed directly onto the plates and incubated overnight at 37ºC. Then, the same procedure was repeated for clinical isolates. The diameter of the inhibition zone was measured and compared to that of the standard strain.
| Antibiotic | Classa | Resistance Rate (%) |
|---|---|---|
| Amikacin | Aminoglycoside | 94.8 |
| Gentamicin | 82.3 | |
| Aztreonam | Beta-lactam | 87.5 |
| Ceftazidime | 60.4 | |
| Piperacillin-Tazobactam | Beta-lactam/ Penicillin | 80.2 |
| Meropenem | Carbapenem | 88.5 |
| Cefepime | Cephalosporin | 80.2 |
| Ciprofloxacin | Fluoroquinolone | 89.6 |
| Norfloxacin | 90.6 | |
| Colistin | Polymyxin | 8.3 |
Antibiotics Used in This Study
3.3. DNA Extraction and Primer Design
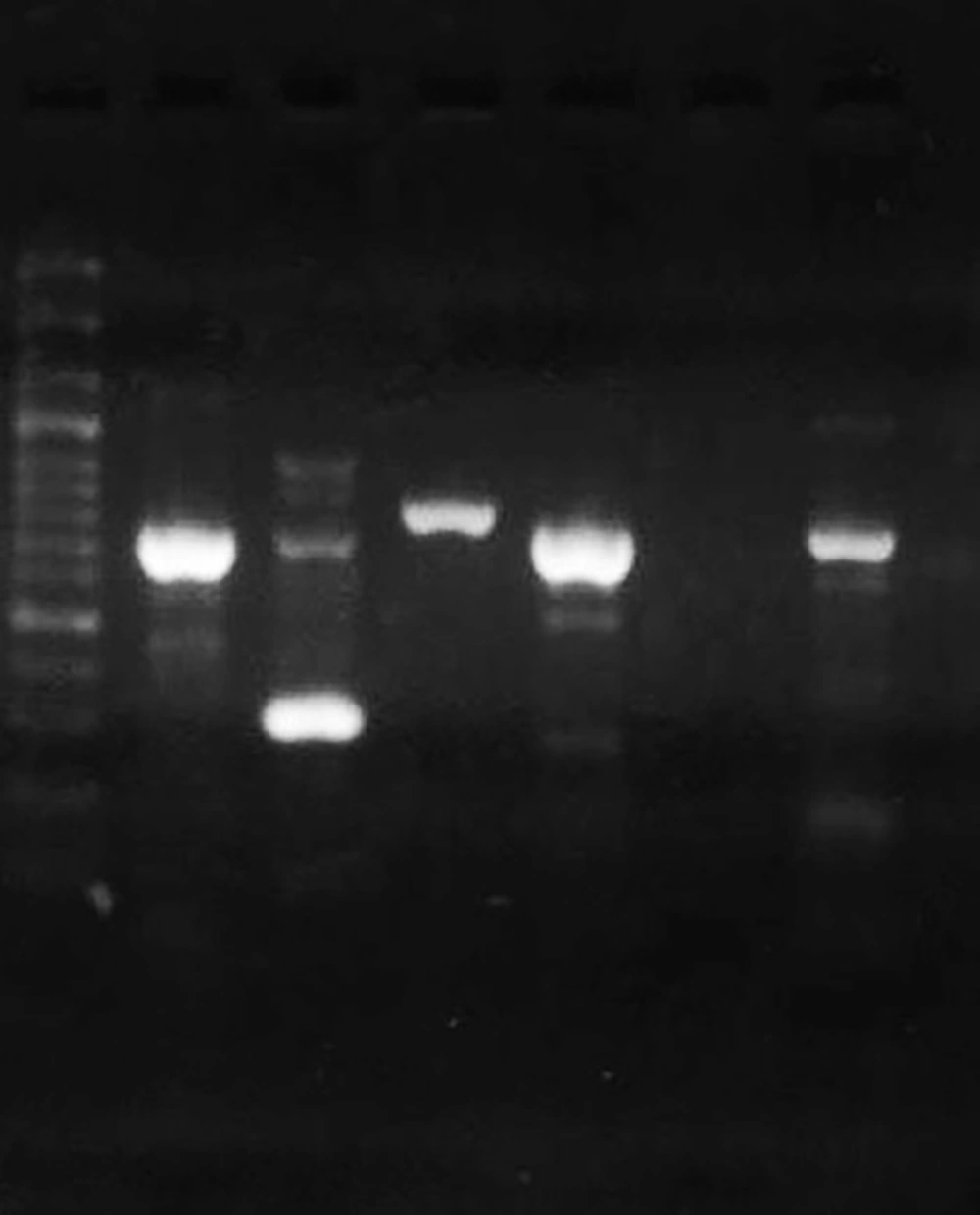
The DNA of clinical isolates and reference strain ATCC 27853 was extracted by the boiling method. The concentration and purity of DNA samples were checked by a Nanodrop spectrophotometer (Thermo Fisher Scientific, US). All isolates were tested for the presence of toxA as a gene that can inhibit eukaryotic protein biosynthesis at the level of polypeptide chain elongation factor 2. The expected PCR product size for toxA was 396 bp (14). The lasR and pqsR genes were 717 bp and 996 bp in length, respectively. Hence, we designed two sets of primers for each gene to cover the entire gene. First, the whole sequences of P. aeruginosa lasR and pqsR genes were extracted from the National Center for Biotechnology Information (NCBI) database. The conserved parts of the genes were selected by MEGA 6 and imported to Gene runner software for designing a set of primers. The specificity of primers was checked using the primer Blast tool in NCBI (10). Then, the primers with the best specificity were chosen and sent to Metabion Company, Germany, for synthesizing. The designed primers are listed in Table 2. The cycling program included one cycle at 94ºC for one minute, 35 cycles of 62ºC for one minute, 72ºC for one minute, and a final elongation step at 72ºC for four minutes. Reference strain P. aeruginosa ATCC 27853 was used as positive control (Figure 2).
| Target Gene | Encoded Protein | Primer Sequence | Amplicon (bp) | Ref. |
|---|---|---|---|---|
| toxA | Exotoxin A | F:5-GACAACGCCCTCAGCATCACCAGC-3 | 396 | (14) |
| R:5-CGCTGGCCCATTCGCTCCAGCGCT-3 | ||||
| lasR1 | Transcriptional regulator | F:5-ATCTTCAGGGGTCGTCGGG-3 | 730 | This study |
| R:5-ATCTCCCAACTGGTCTTGCC-3 | ||||
| lasR2 | F:5-GAAGTGTTGCAGTGGTGCG-3 | 304 | This study | |
| R:5-GGGATAAGCCAATCCTGCGG-3 | ||||
| pqsR1 | F:5-GTCACCCGCTGTTCGACG-3 | 806 | This study | |
| R:5-ACGATCAAGCAGGACAACGC-3 | ||||
| pqsR2 | F:5-GCACGCACTGGTTGAAGC-3 | 733 | This study | |
| R:5-AAACGACGACTCCCCGTGC-3 |
Primers Used in This Study
3.4. Analysis of DNA and Protein Sequences
The PCR products of seven isolates were purified and sequenced by Bioneer Company, Korea. Then, the nucleotide blast program (NCBI) and Clustal Omega program were used to analyze the DNA and protein sequences. The impact of mutations on protein stability and protein function was evaluated by I-Mutant (15), iStable (16), MuPro (17), and PROVEAN (18) webserver. Reference strain PAO1 was selected to compare with.
4. Results
Ninety-six isolates of P. aeruginosa were grown from 120 burn wound samples. After biochemical tests and Gram staining, the final identification was done based on the toxA gene and all the 96 isolates were identified as absolute P. aeruginosa. Some of the clinical isolates showed resistance to several antibiotics, named MDR, XDR, and Pandrug Resistant (PDR), owing to the extreme of their resistance. According to the MDR and XDR definitions, XDR isolates are basically part of MDR isolates. Hence, 95.8% of the isolates were MDR, of which 87.5% were XDR. Since no PDR isolates were seen in this study, only showed 4.2% of the clinical isolates to be susceptible to treatment (Figure 3). Among the antibiotics used in this study, the most effective antibiotic to treat P. aeruginosa infection was colistin from the polymyxin family, whereas the bacterium showed the most resistant rate to amikacin as an aminoglycoside (19). The results are presented in Table 1.
Among the clinical isolates showing resistance to antibiotics, those that had the highest minimum inhibitory concentration (MIC) and were the most resistant isolates, including three MDR and four XDR strains, were selected as the representatives of all isolates and sent for two-way sequencing. All the seven isolates were sequenced for lasR and pqsR genes. The mutations are listed in Table 3. Among these resistant isolates, five and two isolates were devoid of any mutation in the lasR and pqsR genes, respectively. These isolates were completely similar to reference strain PAO1. Amongst the isolates assessed for mutations in the lasR gene, isolate S-5 had two silent mutations in position L36 and N209 and S-2 had one missense mutation in position 180 which led to substitution R180Q. Apart from this missense mutation, three silent mutations in L36, A121, and N214 were observed, as well. On the other hand, the assessment of the pqsR gene showed that isolate S-1 had one silent mutation in R126 and one nonsense mutation in E259. A similar pattern was repeated for S-6 and the mutations were the same as mutations in S-1. Two silent mutations in R126 and E259 were observed for S-3. Also, this isolate had one missense mutation which led to substitution A314V. There were only two silent mutations in L98 and P177 for S-4 while S-5 had three silent mutations in E152, V215, and E219. Generally speaking, the rate of mutations was more in the pqsR gene than in the lasR gene.
| Strain | Resistant Status | LasR Mutation | PqsR Mutation |
|---|---|---|---|
| S-1 | XDR | No mutation | Silent (R126), Nonsense (E259-) |
| S-4 | No mutation | Silent (L98, P177) | |
| S-5 | Silent (L36, N209) | Silent (E152, V215, E219) | |
| S-7 | No mutation | No mutation | |
| S-2 | MDR | Silent (L36, A121, N214), Missense(R180Q) | No mutation |
| S-3 | No mutation | Silent (R126, E259), Missense (A314V) | |
| S-6 | No mutation | Silent (R126), Nonsense (E259-) |
Types of Mutations Seen in the Present Study
Since there are many new methods and algorithms to predict amino acid substitutions and their impacts, it would be better to combine these prediction methods and reach a consensus. Hence, we used three predicting programs, including Mupro, iStable, and I-Mutant, to find out the impact of missense mutations on protein stability observed in the present study. Also, the impact of missense mutations on the biological function of proteins was assessed by PROVEAN. According to Table 4, the only missense mutation in LasR (R180Q) decreased the stability of the LasR protein and had a deleterious impact on protein function, whereas substitution A314V in PqsR led to increased protein stability along with a neutral impact on protein function. The majority of programs could not assess the impact of nonsense mutations, but it is obvious that nonsense mutations could produce truncated and nonfunctional proteins as expected. Even though both missense mutations happened in MDR strains, nonsense mutations were seen in both XDR and MDR isolates.
| Protein | Site | Protein Stability | Protein Function | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mupro | iStable | I-Mutant | Consensus | PROVEAN | ||||||
| ΔΔG | Stability | Score | Stability | ΔΔG | Stability | Score | Prediction | |||
| LasR | R180Q | -1.028 | ↓ | 0.738 | ↓ | -0.86 | ↓ | Decrease | -3.864 | Deleterious |
| PqsR | A314V | 0.241 | ↑ | 0.565 | ↑ | -0.11 | ↓ | Increase | -0.004 | Neutral |
Analysis of Protein Stability and Protein Function
5. Discussion
Microbial drug resistance has become a serious clinical and public health problem. The mechanisms of drug resistance have become a focal point of research in recent years (20). The magnitude of this problem has heightened due to the rapid genetic changes in genes involved in antibiotic resistance even to the most recently developed drugs (21). Mutations in the genes of infectious organisms play a primary role in resistance and this leads to changes in drug interaction with its target protein (22). This scenario has signified the importance of studying mutations in responsible genes.
There is a large body of evidence that mutations in QS systems can interfere with the ability of P. aeruginosa to cause general and local damage in burn wound infection (23). Thereby, mutations in QS genes of P. aeruginosa caused it to lose its pathogenic potential compared to wild type strains (24). This infection can lead to graft loss, prolonged hospital stay, systemic sepsis, and even increased mortality in burn units (25); hence, the colonization rates have increased to 50% among hospitalized patients. Also, prolonged antibiotic therapy makes this scenario worse by the disruption of normal microbial flora (26).
The lasR gene encodes a protein critical for the initiation of QS response involved in virulence factor production and biofilm formation, signifying that other factors controlled by lasR are critical determinants of P. aeruginosa pathogenesis in burn wound infection (27). However, P. aeruginosa strains with mutations in lasR have been predominately isolated from infections and emerged during in vitro evolution (28). In contrast, we found out five silent and one missense mutations in the LasR protein sequence, but five of the clinical isolates were devoid of any mutation, signifying that other mechanisms are probably involved. It seems that the loss of QS regulation as a result of the accumulation of mutations in the key QS regulator, LasR, is especially common among patients with cystic fibrosis (29). Basically, P. aeruginosa acute virulence in diverse model hosts can be reduced as a result of the inactivation of lasR, whereas the loss of LasR function may represent a marker of early-stage chronic infection of the cystic fibrosis airway with clinical implications for antibiotic resistance and disease progression (30). In the present study, silent mutations prevailed over other types of mutations. On the other hand, it is strongly supposed that the inactivation of lasR is probably associated with conferring resistance to antibiotics (31), while we observed only one missense mutation with a detrimental impact on protein sequence. The sequences assessed here were completely resistant to antibiotics so that three of them were MDR and the others were XDR. López-Causapé et al. reported the main antibiotic resistance mutations among which, P117G was dominant (32); it is in contrast to our study, but it should be mentioned that silent mutations in position L36 happened in two clinical isolates.
Bottomley et al. reported atomic interactions between protein LasR and its autoinducer. The amino acid residues involved in these interactions included Tyrosine-56, Arginine-61, Aspartate-73, Threonine-115, and Serine-129. These residues can bind concurrently to cause protein folding, leading to the dimerization of LasR, thus allowing for DNA binding to the promoter and consequent transcriptional activation of QS-controlled genes. Furthermore, Bjarnsholt et al. reported that mutations in Tyrosine-56 and Threonine-75 in the lasR protein would impair autoinducer binding since they strongly interact with the autoinducer (33). In contrast, we did not find any mutation in this region, but we found that missense mutations in region Arginine 180 (R180Q) had a damaging effect on protein function; this also reduced the stability of the protein when compared to strain PAO1. Compared to this missense mutation, silent mutations were observed in positions L36, A121, N209, and N214. It should be noted that several transcriptional regulators, which belong to QS, were expressed at a higher level in PAO1 than in ATCC 27853; moreover, the complete genome of strain ATCC 27853, which usually is used to survey antibiotic susceptibility, is still lacking (34). That is why many studies make comparisons with reference strain PAO1.
In a study, the importance of Gln194 or Tyr258 for PqsR function was assessed by constructing full-length PqsR mutants with mutations at either mentioned positions. The results of this investigation indicated that although Q194E retained at least 88% functionality, this mutant was virtually inactive and almost failed to respond to the Pseudomonas quinolone signal in the pqsA mutant. On the other hand, a hydrophobic amino acid at position 258 is required for PqsR functionality since Y258A mutation renders PqsR inactive (35). No mutations at positions 194 and 258 were seen in our study, but the only missense mutation in the present study (A314V) increased protein stability along with having a neutral impact on protein function. As indicated by previous studies, the mutation of pqsR ends up in the termination of phnAB and pqsABCDE expression, implying PqsR is crucial for PQS signal transduction.
Null mutants of the pqs system decreased biofilm development and reduced the generation of virulence factors, including some enzymes. The pqs system is also required for complete P. aeruginosa virulence toward plants, nematodes, and mice (7). Apart from the missense mutation, there was a nonsense mutation at position 259 (E259-), which was repeated in two isolates. Since the PROVEAN web server cannot assess the impact of nonsense mutations, there is a consensus about nonsense mutations that have a more dramatic change than missense mutations and result in a premature stop codon, produce truncated and typically nonfunctional proteins (36). Furthermore, these nonsense mutations are more likely to have pathogenic consequences due to disrupting protein structure; they have also more disadvantageous effects than nonsynonymous mutations (37, 38).
5.1. Conclusions
In summary, lasR and pqsR possibly can play a key role in antibiotic resistance, but they are not the only factors and environmental elements possibly involved in this matter. Since geographical differences are important in resistance-related mutations and drug efficacy, further investigations with a large number of strains are required. Overall, the accumulation of mutations in a gene is a common reason for antibiotic resistance. Hence, studying mutations is necessary to understand the repertoire of molecular mutations and recognize the conserved and variable residues. Moreover, it helps predict the emergence and spread of resistance mutations in genes and design a promising antibiotic with the ability to overcome antibiotic resistance as much as possible.