1. Background
Burn injury is a common form of injury across the world, statistically more common in non-developed countries due to less preventing strategies (1). Despite remarkable advances in the management of burn injuries, infection is still a considerable problem. For example, a report shows that 73% of burn patients die because of infection within the initial five days of the injury (2). Burn injury dramatically weakens the barrier function of the integumentary system against bacterial pathogens (3). Besides, burnt, necrotic skin provides a suitable environment for colonization and proliferation of pathogenic microorganisms (4). The most frequent and devastating pathogens are Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumonia, and Staphylococcus aureus (5, 6).
Acinetobacter spp. are one of the most common Gram-negative multidrug-resistant (MDR) nosocomial agents. They constitute a major cause of wound infection besides pneumonia, endocarditis, meningitis, and urinary tract infections in hospital settings (7, 8). Acinetobacter species are commonly found in soil and water. They have the ability to survive in harsh environments and even in exposure to various common disinfectants. These features along with their ability to grow at a wide range of temperatures allow them to survive in hospitals, especially Intensive Care Units (ICUs) (2, 9, 10). The MDR strains are defined as isolates resistant to at least three classes of antimicrobial agents (11). Statistics show that about one million people get infected with A. baumannii all around the world annually (12). A one-year study published in 2017 showed that 69% of A. baumannii isolates were MDR in Saudi Arabia. Besides, a recent systematic review and meta-analysis estimated that the pooled prevalence of MDR isolates of A. baumannii is 72% (22.8% to 100%) in Iran annually (13).
In burn patients, Acinetobacter can be transmitted by invasive clinical procedures, such as mechanical ventilation, and indwelling devices, including the central venous (CV) line and urinary catheters (2). Transmission from dust to the exposed wound is another major source of infection acquisition. Therefore, the transmission rate can be reduced if (a) healthcare staff pay proper attention to hand hygiene and consider standard precaution protocols; (b) patients are screened regularly for possible infections; (c) hospital environment is disinfected continuously ; and (d) proper air conditioning is applied to remove dust from the ward (14, 15).
Effective antibiotics against A. baumannii are not limited to carbapenems, cephalosporins, aminoglycosides, colistin (the most effective drug in vitro), and tigecycline, which can be administered alone or in combination regimens (16). Choosing a proper antibiotic for burn wards is a challenge for physicians, especially based on the fact that burn patients are often infected with several bacterial species (multi-bacterial infections).
The remarkable increase in antibiotic resistance among A. baumannii strains and frequent outbreak reports in ICUs have raised a great deal of concern in recent years (17-19). For example, a relatively recent systematic review and meta-analysis showed an increase in carbapenem resistance rates during 2010 - 2011 and 2012 - 2013 (20). This is the case for many other microbial agents found in clinical settings. The prevalence and sensitivity patterns vary from one center to another. Thus, each unit needs to monitor its' own pattern to control nosocomial infections (5, 6). For instance, the prevalence of pandrug-resistant (PDR) and extensively drug-resistant (XDR) isolates of A. baumannii in the central region of Iran has been reported to be 11% and 89%, respectively (21). Such summative data from different units can provide a general view of antibiotic resistance patterns in the region. There are a few studies about the A. baumannii resistance pattern in Iran. However, most of these studies suffer from low sample sizes (16, 22) or they were performed in a short period (22), which may affect the true estimation of outcomes.
2. Objectives
The present study was conducted to assess the prevalence and antibiotic resistance pattern of A. baumannii in the only burn center in northeastern Iran during a three-year period.
3. Methods
3.1. Patient Selection and Study Design
In this cross-sectional study, we assessed all cultures from burn patients who were admitted to the burn center of Imam Reza Hospital in Mashhad between 2012 and 2014. Imam Reza Hospital is a 1,500-bed tertiary university hospital with nearly 40,000 annual admissions. The referral burn center located in the hospital has almost 800 annual admissions for acute burn patients and consists of two burn wards with a totally 50 beds and a Burn ICU (BICU) with 10 beds. All patients were visited every day by a burn team including expert surgeons, specialists of infection diseases, nutritionists, psychologists, and physical therapists. In addition, pediatric patients were visited daily by an expert pediatrician. Decisions on appropriate antibiotic administrations were made by the burn team according to the hospital infection control protocol based on the source of infection, accompanying conditions, lab results (including bacterial identification, antibiogram), patients’ medical conditions, and other minor criteria listed in the infection control policy instructions.
3.2. Culture
Wound cultures were routinely taken from all patients ≥ 24 hours after a burn accident. The wound cultures would be repeated based on clinician decisions at different time-points during the hospitalization period. All wound swabs and biopsy specimens were collected from the marginal tissue of the wound according to the standard sampling protocols. Blood culture and other routine cultures including urine, bronchial secretion, stool, and the centrally inserted CV line were taken just for patients who were highly suspected of infection according to the clinical condition of the patient.
3.3. Bacterial Identification/Antibiogram
Acinetobacter spp. as non-fermentative bacilli were identified based on routine microbiological tests including Gram staining, colony morphology, motility (negative), lactose (positive), indole (negative), oxidase reaction (negative), glucose oxidation, and growth at 44ºC (23). Antibiotic Susceptibility Testing (AST) was performed according to the Clinical and Laboratory Standards Institut (CLSI) instructions based on the disk diffusion method. Briefly, a bacterial suspension with a concentration of 0.5 McFarland was prepared and cultured on Mueller-Hinton Agar (MHA). The antibiogram discs from Rosco Diagnostica (Taastrup, Denmark, www.rosco.dk) were placed on different zones of the culture plate and incubated for 18 - 24 hours at 35ºC - 37ºC. The diameter of growth inhibition was measured and interpreted based on the CLSI protocols (24, 25). For Acinetobacter antimicrobial testing, we routinely used ciprofloxacin (0.5 µg), cefepime (30 µg), ampicillin (33 µg), gentamicin (40 µg), amikacin (40 µg), cefotaxime (30 µg), ceftazidime (30 µg), colistin (10 µg), imipenem (15 µg)/meropenem (10 µg), ceftriaxone (30 µg), co-trimoxazole (1.25 + 23.75 µg), piperacillin (100 µg), and piperacillin-tazobactam (10 µg), all purchased from the above-mentioned company.
3.4. Statistical Analysis
Data were checked for possible errors. The statistical Package for Social Sciences (SPSS Inc. Release 2007; SPSS for Windows, version 16.0. Chicago, SPSS Inc.) was used for data analyses including descriptive (frequency and percentage) and inferential (chi-square) analyses. All tests were two-tailed and P values of less than 0.05 were considered statistically significant.
4. Results
A total number of 5,080 samples were studied among which, 75.3% were positive for bacterial growth. Nearly half of the positive cultures (51.9%, n = 1985) were positive for Acinetobacter spp. The most frequent positive cultures for A. baumannii were observed in wound samples (Table 1). The resistance rate of Acinetobacter spp. against the employed antibiotics varied from 0.9% for colistin to 100% for piperacillin-tazobactam. Some of these antibiotics such as ampicillin, imipenem, and meropenem were nearly completely ineffective against Acinetobacter spp. in vitro due to the high resistance rates (from 94% to 97%) (Table 2). It can be concluded that all Acinetobacter spp. were MDR due to considerable resistance to fluoroquinolones (95%), cephalosporins (93% - 98%), penicillins (97%), carbapenems (94% - 95%), and beta-lactamase inhibitors (87% - 100%).
| Number of Cultures | Number of Positive Cultures (%) | Number of Acinetobacter-Positive Cultures (%) | |
|---|---|---|---|
| Type of Culture | |||
| Blood | 376 | 218 (57.7) | 83 (38.2) |
| Wound | 4608 | 3558 (77.2) | 1883 (52.9) |
| Other | 96 | 49 (50.5) | 19 (37.5) |
| Location | |||
| Burn ward | 3917 | 2806 (71.6) | 1507 (53.7) |
| Burn ICU | 1163 | 1019 (87.5) | 478 (46.8) |
| Total | 5080 | 3825 (75.3) | 1985 (51.9) |
aValues are expressed as No. (%).
| Antibiotic Class/Name | Resistance | Intermediate | Susceptible |
|---|---|---|---|
| Fluoroquinolones | |||
| Ciprofloxacin | 887 (95.2) | 11 (1.2) | 34 (3.6) |
| Cephalosporins | |||
| Cefepime | 1108 (93.4) | 11 (0.9) | 67 (5.6) |
| Cefotaxime | 54 (93.1) | 0 (0) | 4 (6.9) |
| Ceftazidime | 301 (95.6) | 2 (0.6) | 12 (3.8) |
| Ceftriaxone | 130 (97.7) | 1 (0.8) | 2 (1.5) |
| Penicillins | |||
| Ampicillin | 29 (96.7) | 0 (0) | 1 (3.3) |
| Aminoglycoside | |||
| Gentamicin | 464 (52.2) | 14 (0.7) | 411 (46.2) |
| Amikacin | 1458 (88.7) | 63 (3.8) | 122 (7.4) |
| Polymyxins | |||
| Colistin | 16 (0.9) | 0 (0) | 1803 (99.1) |
| Carbapenems | |||
| Imipenem | 950 (94.4) | 4 (0.4) | 52 (5.2) |
| Meropenem | 706 (95.0) | 5 (0.7) | 32 (4.3) |
| Sulfonamides | |||
| Co-trimoxazole | 1 (50) | 0 (0) | 1 (50) |
| Beta-lactamase inhibitors | |||
| Piperacillin | 7 (87.5) | 1 (12.5) | 0 (0) |
| Piperacillin-tazobactam | 8 (100) | 0 (0) | 0 (0) |
aValues are expressed as No. (%).
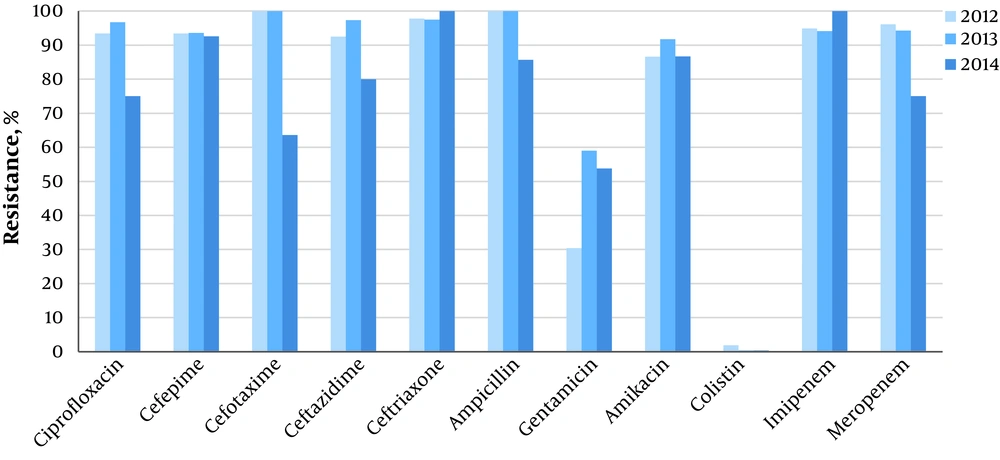
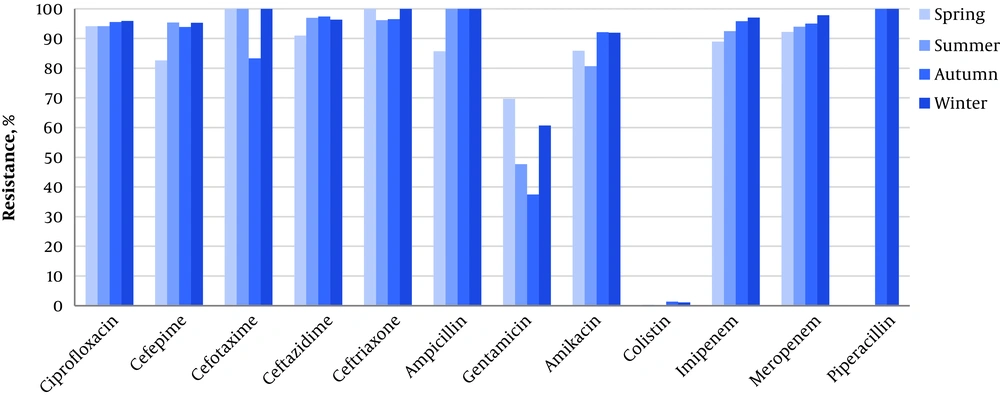
As Table 3 shows, there were no statistically significant relationships between the resistance pattern and patients’ gender, culture type, and burn subdivisions. However, we found a significant difference in the resistance rate to gentamicin between the units (64% in burn wards vs. 36% in BICU, P < 0.001). We also investigated the chronological pattern of antibiotic resistance. It was revealed that although some decremental or incremental changes existed in different study years and seasons, the majority of these variations were not statistically significant. The only identified significant difference in resistance rate was related to amikacin among different years (P < 0.001) and different seasons. The highest rate of resistance to amikacin was observed in autumn (92.1%, P < 0.001) (Figures 1 and 2). except that, an instant pattern was observed along studied years. The resistance rate to colistin decreased from 1.9% in 2012 to 0.4% in 2014.
| Antibacterial Agent | Male (N = 1062) | Female (N = 923) | P Value | Burn Ward (N = 1508) | Burn ICU (N = 477) | P Value | Wound Culture (N = 1884) | Blood Culture (N = 83) | Other Cultures (N = 18) | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | 455 (51.3) | 432 (48.7) | 0.85 | 676 (76.2) | 211 (23.8) | 0.12 | 832 (93.8) | 48 (5.4) | 7 (0.8) | -b |
| Cefepime | 580 (52.3) | 528 (47.7) | 0.55 | 908 (81.9) | 200 (18.1) | 0.05 | 1035 (93.4) | 61 (5.5) | 12 (1.1) | - |
| Cefotaxime | 25 (46.3) | 29 (53.7) | 0.11 | 43 (79.6) | 11 (24.4) | > 0.99 | 49 (90.7) | 5 (9.3) | - | > 0.99 |
| Ceftazidime | 170 (56.5) | 131 (43.5) | - | 206 (68.4) | 95 (31.6) | - | 278 (92.4) | 18 (6.0) | 5 (1.7) | - |
| Ceftriaxone | 57 (43.8) | 73 (56.2) | - | 95 (73.1) | 35 (26.9) | - | 122 (93.8) | 7 (5.4) | 1 (0.8) | - |
| Ampicillin | 12 (41.4) | 17 (58.6) | 0.43 | 25 (86.2) | 4 (13.8) | 0.16 | 28 (96.6) | 1 (3.4) | 0 (0) | > 0.99 |
| Gentamicin | 257 (55.4) | 207 (44.6) | 0.31 | 297 (64.0) | 167 (36.0) | < 0.001 | 440 (94.8) | 18 (3.9) | 6 (1.3) | - |
| Amikacin | 796 (54.6) | 662 (45.4) | 0.60 | 1070 (73.4) | 388 (26.6) | 0.13 | 1394 (95.6) | 49 (3.4) | 15 (1.0) | - |
| Colistin | 9 (56.3) | 7 (43.8) | - | 15 (93.8) | 1 (6.3) | - | 16 (100) | 0 (0) | 0 (0) | - |
| Imipenem | 507 (53.4) | 443 (46.6) | - | 738 (77.7) | 212 (22.3) | - | 894 (94.1) | 46 (4.8) | 10 (1.1) | - |
| Meropenem | 371 (52.5) | 335 (47.5) | - | 603 (85.4) | 103 (14.6) | - | 664 (94.1) | 37 (5.2) | 5 (0.7) | - |
| Co-trimoxazole | - | 1 (100) | - | 1 (100) | 0 (0) | > 0.99 | 1 (100) | - | - | - |
| Piperacillin | 6 (85.7) | 1 (14.3) | > 0.99 | 6 (85.7) | 1 (14.3) | - | 7 (100) | - | - | - |
| Piperacillin-Tazobactam | 4 (50) | 4 (50) | - | 7 (87.5) | 1 (12.5) | - | 8 (100) | - | - | - |
aValues are expressed as No. (%).
bChi-squared test could not be performed.
5. Discussion
Despite remarkable improvements in burn patients’ management, nosocomial infections caused by bacterial pathogens still remain a major cause of morbidity and mortality among these patients (26). In addition to BICU environmental conditions including high humidity and temperature, the critical conditions of admitted patients are among the factors predisposing to high infection rate in burn patients, including higher burn total body surface area (TBSA), inhalational injury, multi-organ damage, nutritional insufficiency, impaired immune function, delay in surgical intervention due to unstable vital status, and other weakening factors (27). The control of A. baumannii as a very common cause of infection among burn patients has been a laborious process, especially in developing countries, mostly because of MDR strains (28). The great challenge is to select the most effective antibiotic(s) to cope with these infections (29). The challenge is even worse when multi-bacterial infections exist, which is a common condition in burn units. In this study, we aimed to investigate the resistance pattern and possible associations between resistance patterns and gender, year, culture type, and the unit in which patients were hospitalized (BICU vs. burn wards).
Similar to Pseudomonas, Acinetobacter has low nutritional requirements and as a heterotrophic and chemoheterotrophic organism, it can provide its needs from the environment. It can be found in water, soil, dust, and sewage, and is frequently detected in healthcare settings. This pathogen can consume a variety of carbonized sources and can grow in a wide range of temperatures and pH environments. Although the pathogen is not considered a biofilm/spore-producing pathogen, due to minor nutritional requirements, it has high viability even in harsh environments (27). Different studies have reported high resistance of A. baumannii to the majority of antibiotics, most notably amikacin (46.7% to 97.6%), imipenem (94.4%), meropenem (95.0%), and cefepime (93.4%) (2, 5, 16).
In our study, nearly half of the tested isolates were susceptible to gentamicin and high susceptibility was observed to colistin (99%). A preliminary study was performed by Sarhaddi et al. (16) on 54 isolates of A. baumannii from burn patients in Imam Reza Hospital from January to December 2014. We extended the study with larger sample size and a longer period of study to monitor possible changes in antibiotic resistance patterns among Acinetobacter spp. While in the aforementioned study, the resistance rates to carbapenems and amikacin were similar to our study, none of the isolates was resistant to colistin. This difference can be related to the small sample size of their study or most importantly the emergence of strains resistant to colistin, which should be considered as an alarm. Lab technical errors that might be observed in a large sample size might be another explanation that should not be ignored. In general, the resistance rate to colistin ranged from 0% to 19% in different burn centers across different parts of the country (28, 30). The emerging resistance to colistin should be considered as a worrisome threat because colistin is one of the few remaining antimicrobial choices for A. baumannii.
In our study, imipenem and meropenem were nearly ineffective against A. baumannii. However, previous studies showed contrary results. In a study conducted in Tehran between July 2006 and December 2007 (8), 52.5% of isolates were resistant to meropenem and the resistance rate to imipenem was almost the same. Similarly, in another study, imipenem was ineffective against 50.9% of isolates in Shahid Zare Hospital, Sari, Iran (31). In a wider screen, Bowo and Puntri found that A. baumannii was susceptible to meropenem in 42.9% of isolates in a BICU in Indonesia (6). The increasing resistance to carbapenems has been reported at the beginning of the third millennium for various bacteria (32). In a study conducted in New Delhi, the resistance rates of Acinetobacter spp. were 96.2% and 97.6% to gentamicin and amikacin, respectively, between 2010 and 2014 (2). High resistance to gentamicin was also reported in other studies (16, 28, 30, 31). The antibiotic prescription policy is widely different in burn centers; thus, resistance patterns vary between different clinical settings and it is recommended that each unit performs its own survey to monitor the resistance rate and pattern changes over time.
The year-by-year analysis showed that just resistance to gentamicin and amikacin changed during the three-year study period. The resistance pattern of A. baumannii for amikacin also had significant changes in four seasons. Based on a previous report (33), we expected to have an increase in prevalence, incidence, and drug resistance during the summer, but the drug resistance to amikacin decreased during the summer. This can be explained by variations in bacterial strains that may be due to incoming tourists and pilgrims to Mashhad during the summer. Based on our findings, despite the higher rate of resistance in burn wards, there was no significant difference between BICU and burn wards in the resistance rate of A. baumannii against most of the applied antibiotics. However, gentamicin had a resistance rate of 86.2% in burn wards and 13.8% in BICU, indicating that wards were colonized with strains resistant to gentamycin or the gentamicin resistance gene was widely spread among bacteria in the ward environment.
Besides, the difference between Burn wards and BICU was very close to significance for cefepime. In a study by Uwingabiye et al., the results were in contrast to our results so that the resistance rate was significantly higher in BICU than in wards (19). This difference can be related to environmental differences between hospitals such as the level of hygiene in ICUs and the proficiency of personnel in different wards (34). Different levels of hand hygiene and environmental disinfection can change the results. Thus, hand transmission can play a significant role in spreading pathogens (35). Also, a study by Gales et al. showed a higher prevalence of infection in ICUs (36). One possible explanation is the presence of persistent endemic clones in ICUs although further research such as multicenter intensive environmental sampling studies and comparison of infection policies and building characteristics of wards is needed to explore the topic more closely (37, 38).
The limitation of our study was that we did not include the clinical outcome of patients, though one should note that some confounding factors such as age and burn TBSA play roles in the outcome of the patients’ clinical condition and we cannot entirely link the outcome to drug-resistant strains. It should be noted that in collecting samples, we could not differentiate between active infection and colonized cases. This is while the awareness of the prevalence and patterns of antibiotic resistance in cases of colonization might have epidemiological importance due to the link between these strains and environmental bacterial contamination. Besides, we used the disk diffusion method for analyzing colistin resistance, which showed 1% resistance. However, one could speculate that this might be due to random technical errors in the laboratory.
Another limitation that should be noted is that we did not identify A. baumannii species based on the PCR technique. However, most Acinetobacter spp. are A. baumannii in clinical settings and it is unlikely that a very limited percentage of other species can entirely affect our interpretations; thus, the results could be logically linked to A. baumannii. Also, the present study evaluated the phenotypic disk diffusion method routinely performed in a clinical setting that could be extended to MIC evaluations and genotypic studies. Regardless of the aforementioned limitations, the results of the present study are important because of presenting updated data with large sample size and a three-year period of the study. Upcoming studies are proposed to evaluate the environmental contaminations and comparing the antibiotic resistance patterns of such bacteria with the patients’ isolated bacteria. Besides, molecular investigations to find resistance genes might be helpful.
5.1. Conclusions
The high MDR rate in A. baumannii isolates in the studied burn center suggests that local antibiotic prescription policies should be revised and infection control policies should be improved. Also, antibiotic cycling and restrict infection control strategies should be implemented in high-risk wards such as burn units.