1. Background
Helicobacter pylori is one of the most widely distributed and most common bacteria in the world (1). China is a country with a high incidence of H. pylori infection. The infection rate is 40 - 90% among the general population of China with an average rate of 58.07% (2). According to statistics, approximately 700 million people are infected with H. pylori in China (3). Helicobacter pylori infection is an important cause of digestive tract diseases such as chronic gastritis, digestive ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma. It is also related to parenteral diseases such as blood diseases, cardiovascular diseases, autoimmune diseases, and skin diseases (4). Helicobacter pylori infection is associated with several common gastrointestinal diseases such as peptic ulcer, gastric precancerous lesions, and others. This infection is also related to a variety of other systemic diseases such as cardiovascular disease and diabetes. In 1994, Mendall (5) first proposed that H. pylori infection had a certain correlation with the occurrence of coronary atherosclerotic heart disease.
Helicobacter pylori infection is in the association with geographic area and socioeconomic level. The H. pylori infection rate is lower in developed countries than in developing countries (6) so that the H. pylori infection rate is generally lower than 30% in developed countries, while its infection rate might be as high as 50 - 70% in developing countries. In a randomly selected sample of 930 adults aged 35 - 74 years from four areas of Italy, the H. pylori infection rate was 45% (7). In the UK, the H. pylori infection rate was 37% in male employees. In the United States, the H. pylori infection rate in the general population is approximately 30 - 40%.
Some studies have pointed out that the poorer economic status and a lower degree of culture may induce higher H. pylori infection rates (8). Helicobacter pylori infection has become a public health problem that seriously endangers the health of residents in China. There may be large differences in the rate of H. pylori infection among various sectors of an area (8).
2. Objectives
We aimed to investigate and analyze the epidemic trend of H. pylori infection and its related factors in the Tianjin Binhai area to provide a scientific basis for the prevention of H. pylori infection in this area.
3. Methods
3.1. Patients and Criteria
To understand the current status of H. pylori infection in the Tianjin Binhai area and to evaluate the factors related to H. pylori infection, we conducted a study from January 2013 to July 2013. A total of 10 cluster sampling surveys were performed in the development zone, the urban zone and suburban zone of Tianjin Binhai area. In total, 2235 cases (835 males and 1400 females) were invited to participate in the study; however, 2128 cases were enrolled in the survey, with a response rate of 95.2%. The age range was 4 to 90-years-old, comprising 1985 adults (≥ 18 years) and 143 children (≤ 18 years). We excluded people with antibiotics or acid inhibitors use in the last three weeks, the previous diagnosis of cancer, a history of gastrectomy, and the age of ≤ 18 years. People from the same household were also excluded.
3.2. Survey Method
A questionnaire was developed by medical specialists according to the “National Helicobacter pylori bacterial epidemiological survey program”. The questionnaire included general data such as gender, age, marital status, education level, occupation, income, residence areas, living habits, or habits and ways of life, formerly medical history, and family history.
3.3. Helicobacter pylori Detection Method
The 14C urea breath test (14C-UBT) was generally adopted and the 13C urea breath test (13C-UBT) was used in children and pregnant groups. We employed HUBT-20 and SN3357 types of H. pylori detection instruments (Shenzhen Zhonghe Haidewei Biotechnology Co., Ltd.) and 14C and 13C urea breath test kits. The 14C-UBT was performed as described previously (9). For the present study, a 14C-UBT value of > 100 dpm/mmol CO2 was defined as positive and < 100 dpm/mmol CO2 as negative. In the morning following an overnight fast, subjects rinsed their mouths prior to the test. Each subject swallowed one C14 urea capsule with lukewarm water. After 25 minutes, subjects were asked to exhale through a straw into a CO2 collector. We recorded the time required for the colored fluid in the CO2 collector to change to colorless. Samples were sealed with caps after adding diluted scintillation fluid using pure methanol-rinsed transfer pipettes. Samples were shaken and mixed well and C14 radiation was measured. For C13-UBT, the subject with overnight fast was asked to exhale into a CO2 collector sample. Then, the subject swallowed one C13 urea capsule, and exhaled into another CO2 collector sample. The two samples were attached to SN3357 types of H. pylori instruments for the detection. The values of dpm were then calculated and data were analyzed as mean ± SD of dpm values.
3.4. Criteria for Diagnosis of Helicobacter pylori Infection
Cases with positive 14C-UBT or 13C-UBT detection results were assessed as current H. pylori infection.
3.5. Statistical Methods
The rate of H. pylori infection was expressed in percentages and analyzed with SPSS V. 19.0 software. Categorical data are presented as proportions. Groups were compared with the chi-square test. Unadjusted and stepwise multivariate Cox regression models were used to assess the effects of qualitative variables on the risk of H. pylori infection. This analysis implies a possible bias or lack of generalization of these tertiles to other patient groups. P < 0.05 was considered statistically significant.
4. Results
4.1. Helicobacter pylori Infection Status
Overall, 1860 adult cases were ultimately enrolled in the study, excluding those with antibiotics or acid inhibitors use in the last three weeks, the previous diagnosis of cancer, and history of gastrectomy. These cases were involved in the data analysis of H. pylori infection rates and risk factors. They comprised 560 (30.1%) male cases and 1300 (69.9%) female cases. The age range was 18 to 90-years-old. There were 858 H. pylori-positive cases in the sample, giving an overall H. pylori infection rate of 46.1%. The infection rate was slightly higher in males than in females (49.2% vs. 44.8%, P = 0.01). Furthermore, the H. pylori infection rate was different in different age groups. The under 20-year-old group had the lowest H. pylori infection rate (19.6%), while it was the highest in the 31 - 40-year-old group and 71 - 80-year-old group (52.4% and 52.5%, respectively). This was followed by the 41 - 50-year-old group, 51 - 60-year-old group, and 61 - 70-year-old group (46.9%, 46.3%, and 47.3%, respectively) (Table 1).
| Item | Cases | Cases of Positive Hp | Rate of Positive Hp (%) | χ2 | P Value |
|---|---|---|---|---|---|
| Gender | 5.897 | 0.01 | |||
| Male | 560 | 276 | 49.2 | ||
| Female | 1300 | 582 | 44.8 | ||
| Total | 1860 | 858 | 46.1 | ||
| Age, y | 39.238 | 0.000 | |||
| < 20 | 58 | 11 | 19.6 | ||
| 21 - 30 | 168 | 63 | 37.6 | ||
| 31 - 40 | 270 | 141 | 52.4 | ||
| 41 - 50 | 380 | 178 | 46.9 | ||
| 51 - 60 | 460 | 213 | 46.3 | ||
| 61 - 70 | 362 | 171 | 47.3 | ||
| 71 - 80 | 136 | 71 | 52.5 | ||
| > 80 | 26 | 10 | 31.2 | ||
| Total | 1860 | 858 |
4.2. The Effect of Socioeconomic Status on Helicobacter pylori Infection
We evaluated H. pylori infection in populations with different socioeconomic statuses in terms of marriage, education, occupation, family income, and residence area were evaluated in the questionnaire survey. The results showed that socioeconomic status had a significant effect on H. pylori infection. Married people had a slightly higher infection rate of H. pylori than unmarried people (47.1% vs. 27.3%, P < 0.01). Helicobacter pylori infection rates were 52.9%, 64.8%, 55%, and 43.5% migrant workers, fishermen, cases without a job, and general staff, respectively, which were higher than the rates in cases with other occupations. Furthermore, students and retirees had lower infection rates (22.0% and 37.6%, respectively). The differences were statistically significant compared to other groups. Helicobacter pylori infection rate was lower in the population with a monthly income of over 3000 Yuan than in people with under 3000 Yuan income level, and the difference was statistically significant (38.3% vs. 48.9%, P = 0.042). Among the study areas, the H. pylori infection rate was the highest in Hangu (57.1%). Furthermore, the population in the Kangcui area had the lowest H. pylori infection rate (38.3%) (Table 2).
| Items | Cases | Cases of Positive Hp | Rate of Positive Hp (%) | χ2 | P Value |
|---|---|---|---|---|---|
| Education levels | 19.436 | 0.000 | |||
| Primary school or below | 586 | 314 | 53.5 | ||
| Junior high school, high school or technical secondary school | 720 | 335 | 46.5 | ||
| Junior college or above | 554 | 209 | 37.7 | ||
| Marital status | 30.156 | 0.000 | |||
| Unmarried | 95 | 26 | 27.3 | ||
| Married | 1765 | 832 | 47.1 | ||
| Occupation | 47.325 | 0.000 | |||
| None | 20 | 11 | 55.0 | ||
| Farmer | 567 | 259 | 45.7 | ||
| Farmworker | 70 | 37 | 52.9 | ||
| Worker | 458 | 225 | 49.1 | ||
| Clerka | 152 | 80 | 52.6 | ||
| Medical staff | 46 | 20 | 43.5 | ||
| Teacher | 160 | 65 | 40.6 | ||
| Student | 50 | 11 | 22.0 | ||
| Fisherman | 88 | 57 | 64.8 | ||
| Retiree | 170 | 64 | 37.6 | ||
| Othersb | 79 | 29 | 36.7 | ||
| Location | 112.438 | 0.000 | |||
| Dagang | 400 | 201 | 50.3 | ||
| Hangu | 254 | 145 | 57.1 | ||
| Hujiayuan | 265 | 101 | 38.1 | ||
| Jixiao | 87 | 30 | 34.5 | ||
| Kangcui | 235 | 90 | 38.3 | ||
| Longsheng Park | 150 | 82 | 54.7 | ||
| Ningchegu | 201 | 98 | 48.7 | ||
| Shanghaiyuan | 118 | 47 | 39.8 | ||
| Tanggu Experiment school | 123 | 54 | 43.9 | ||
| Othersc | 27 | 10 | 37.0 | ||
| Monthly Income | 4.235 | 0.042 | |||
| < 3000 | 1370 | 670 | 48.9 | ||
| > 3000 | 490 | 188 | 38.3 |
aMainly means corporate clerk.
bIncludes seamen, civil servant, driver, self-employed entrepreneur, and so on.
cMeans outpatient visits or volunteers of Hp examination to hospital.
4.3. The Effect of Personal Lifestyle or Habit on Helicobacter pylori Infection
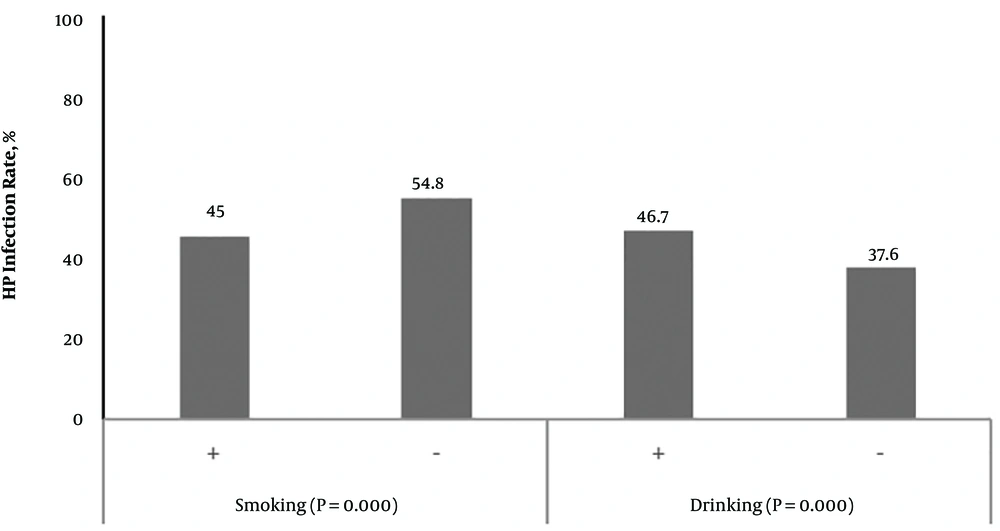
In this survey, we evaluated the relationship between the individual lifestyle or habits and H. pylori infection. The results showed that the H. pylori infection rate was higher in the smoking population than in a normal population (54.8% vs. 45.0%, P < 0.01). The H. pylori infection rate was higher in the drinking population than in a normal population (46.7% vs. 37.6%, P < 0.01) (Figure 1). Furthermore, the H. pylori infection rate was higher in the population with the habit of outside dining (54.7% vs. 48.2%, P = 0.038). This rate was also higher in populations that often ate seafood, drink tea, and ate pickled food. In addition, this rate was higher in populations with a large amount of salt intake (52.4% vs. 45.3%, P = 0.001). Helicobacter pylori infection had no correlation with the consumption of soy products and fresh garlic, vinegar and spicy food. In addition, the rate of H. pylori infection was higher in populations who reared animals at home (52.6% vs. 45.6 %, P = 0.032) (Table 3).
| Items | Cases | Cases of Positive Hp | Rate of Positive Hp (%) | χ2 | P Value |
|---|---|---|---|---|---|
| Drinking tea | 4.357 | 0.018 | |||
| Occasionally | 1721 | 802 | 46.6 | ||
| Frequently | 139 | 56 | 40.3 | ||
| Eating vinegar | 0.638 | 0.210 | |||
| Occasionally | 1698 | 796 | 46.9 | ||
| Frequently | 162 | 62 | 38.3 | ||
| Eating uncooked garlic | 1.453 | 0.214 | |||
| Occasionally | 1711 | 786 | 45.9 | ||
| Frequently | 149 | 72 | 48.3 | ||
| Eating seafood | 19.231 | 0.000 | |||
| Occasionally | 1452 | 615 | 42.3 | ||
| Frequently | 408 | 243 | 59.6 | ||
| Eating beans | 0.985 | 0.125 | |||
| Occasionally | 1645 | 749 | 45.5 | ||
| Frequently | 215 | 109 | 50.7 | ||
| Eating spicy food | 0.894 | 0.389 | |||
| Occasionally | 1523 | 691 | 45.3 | ||
| Frequently | 337 | 167 | 49.6 | ||
| Eating out | 5.123 | 0.038 | |||
| Occasionally | 1621 | 782 | 48.2 | ||
| Frequently | 139 | 76 | 54.7 | ||
| Eating Pickled food | 11.125 | 0.001 | |||
| Occasionally | 1548 | 735 | 47.5 | ||
| Frequently | 212 | 123 | 58.0 | ||
| Eating salt | 11.543 | 0.001 | |||
| Lightly | 1635 | 740 | 45.3 | ||
| Heavily | 225 | 118 | 52.4 | ||
| Keeping animals | 4.453 | 0.032 | |||
| No | 1708 | 778 | 45.6 | ||
| Yes | 152 | 80 | 52.6 |
4.4. The Effect of Personal Health Status on Helicobacter pylori Infection
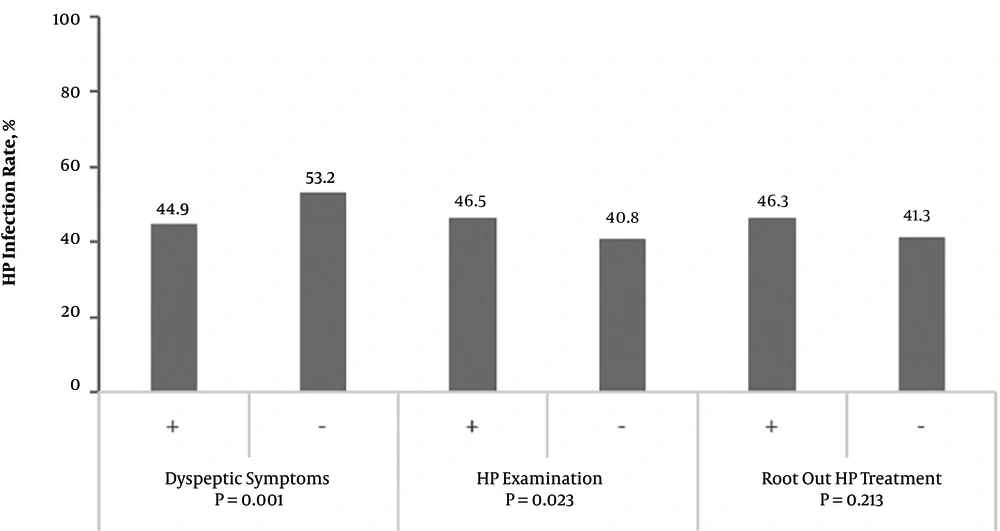
It was found in the survey that 574 cases had one or several kinds of dyspeptic symptoms such as abdominal distension, heartburn, acid regurgitation, nausea, belching, hiccup, and early satiety for nearly a year. The H. pylori infection rate was higher in this population than in the asymptomatic population (53.2% vs. 44.9%, P = 0.001). However, there was no difference in the infection rate between a population who underwent previous H. pylori eradication treatment and the general population. Furthermore, there was no difference in the infection rate between a population with a history of medication in the past three months (including antihypertensive drugs, diabetes drugs, and heart disease drugs) and the general population (Figure 2).
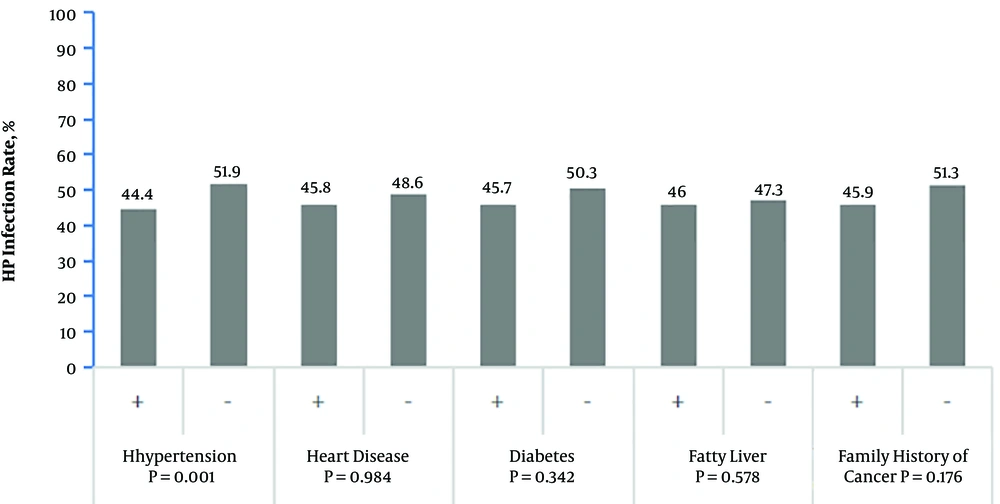
In the survey, there were 422 cases, 155 cases, 208 cases, and 171 cases infected with H. pylori who had a history of hypertension, diabetes, coronary atherosclerosis, and fatty liver, respectively. The H. pylori infection rate was higher in the hypertension population than in the general population (51.9% vs. 44.4%, P = 0.001). There was no significant difference in the infection rate between a population with other underlying diseases and the general population. The family history of cancer was not related to H. pylori infection (51.3% vs. 45.9%, P = 0.76) (Table 3, Figure 3).
4.5. The Effect of Single Testing Factor on Helicobacter pylori Infection
Multivariate logistic regression analysis was performed on possible risk factors for H. pylori infection in the univariate analysis. The results revealed (Table 4) that marriage and education level were important factors affecting H. pylori infection. Furthermore, gender and hypertension may be associated with H. pylori infection. Helicobacter pylori infection was negatively correlated with a previous examination for H. pylori infection.
| Risk Factors | Regression Coefficient | S.E | χ2 | P Value | Exp (B) |
|---|---|---|---|---|---|
| Gender | 0.211 | 0.114 | 3.437 | 0.064 | 1.235 |
| Marriage | 0.937 | 0.226 | 17.130 | 0.000 | 2.552 |
| Digestive tract symptoms or not | 0.184 | 0.104 | 3.142 | 0.076 | 1.202 |
| Smoking | 0.126 | 0.117 | 1.165 | 0.280 | 1.135 |
| Drinking wine | 0.063 | 0.152 | 0.173 | 0.678 | 1.065 |
| Drinking tea | 0.104 | 0.158 | 0.436 | 0.509 | 1.110 |
| Eating seafood | 0.124 | 0.110 | 1.263 | 0.261 | 1.132 |
| Eating out | 0.200 | 0.155 | 1.665 | 0.197 | 1.222 |
| Eating pickled food | 0.208 | 0.139 | 2.249 | 0.134 | 1.231 |
| Eating salt | 0.041 | 0.101 | 0.164 | 0.686 | 1.042 |
| Hypertension | 0.194 | 0.112 | 2.965 | 0.085 | 1.214 |
| Economic status | 0.119 | 0.127 | 0.867 | 0.352 | 1.126 |
| Keeping animals | 0.140 | 0.146 | 0.927 | 0.336 | 1.151 |
| Hp test | -0.484 | 0.187 | 6.732 | 0.009 | 0.616 |
| Age bracket | -0.052 | 0.040 | 1.698 | 0.193 | 0.950 |
| Culture | -0.194 | 0.082 | 5.638 | 0.018 | 0.823 |
| District | 0.034 | 0.018 | 3.712 | 0.054 | 1.035 |
| Occupation | -0.005 | 0.019 | 0.057 | 0.811 | 0.995 |
5. Discussion
5.1. Helicobacter pylori Infection Status
There are significant differences in the H. pylori infection rate among different regions and countries. China is a country with a vast territory and there are significant differences in topographic and geographic conditions. Furthermore, there is a significant difference in the H. pylori infection rate across the country. The region with the lowest infection rate was Guangdong province (42%), and the region with the highest infection rate was Tibetan Lama (90%) (8). The infection rate was 45.39% in Hebei province. In this study, the positive rate of H. pylori infection was 48.6% in 1860 adults in the Tianjin Binhai area, which was lower than the domestic average level and a little higher than the average infection rate in Hebei province. Since H. pylori infection is associated with socioeconomic, health, family, and environmental factors, the incidence rate varies in different areas, even at different times (10, 11) in the same area. Therefore, we can speculate that local, social, economic, and development factors, population density, and lifestyle have important influences on the detection rate of H. pylori.
5.2. The Relationship Between Helicobacter pylori Infection and Gender or Age
In this study, the H. pylori infection rates were 49.2% and 44.8% in men and women, respectively. The rate of male cases was slightly higher than that of female cases, which was not consistent with some reports (12, 13) and consistent with other reports (14, 15). These might be related to the differences in races, living habits, and living conditions of the subjects. In addition, the reason that the positive rate of male cases was higher than that of female cases may be related to the high intensity of work, more opportunities of outdoor dining, smoking, drinking, and ignoring the dietetic hygiene.
Many studies (16, 17) have shown that the H. pylori infection rate exhibited a gradually increasing trend with the increase in age. In this study, it was shown that the H. pylori infection rate in the 31 - 80-year-old group was higher than the average level, which may be due to the relatively frequent social activities, interpersonal communications, and greatly increased opportunities of interpersonal contact transmission. Furthermore, there was an obvious upward trend in the 31 - 40-year-old group than in the 21 - 30-year-old group. These might be due to greater working pressure, lack of regularity of life, and more dinner for social intercourse, which resulted in a higher chance of infection. Thus, prevention should be made early. Good personal hygiene habits should be developed and diet should be monitored to reduce the H. pylori infection. With the further increase in age, the infection rate of people over 80 years decreased (31.2%), which may be due to medications, decreased social activities, and more attention given to the health and diet by the elderly people. In addition, due to the small sample size in this group, there might be deviations in the statistical results. Thus, further studies with larger sample sizes should be conducted.
5.3. The Relationship Between Helicobacter pylori Infection and Personal Lifestyle or Habits
Studies have reported that drinking unboiled water and eating raw vegetables and fruits may increase the H. pylori infection rate (18-20). The results of the present study revealed that the H. pylori-positive rate increased in populations who often consumed seafood, which may be associated with eating raw seafood and increased exposure to contaminated seafood. In this investigation, dogs are the main home-reared animals, and it was found that the positive rate of H. pylori infection was higher in populations rearing dogs. Epidemiological data revealed that rearing dogs is a risk factor for H. pylori infection (21) and it was inferred that dogs may be the potential storage source of bacteria.
In this study, the H. pylori infection rate was higher in smoking and drinking populations. This is consistent with some reports that H. pylori was positively related to smoking and drinking (22), while it contradicts some other research that smoking and drinking were negatively correlated with the H. pylori infection rate (23, 24).
We found that the regular consumption of raw garlic and soy products did not reduce the infection rate of H. pylori. This was different from a previous report that garlic could prevent H. pylori infection (25). However, occasional tea-drinking may reduce the incidence of H. pylori infection that might be induced by catechins found in the tea (26-28). However, frequent tea-drinking would increase the H. pylori infection rate. A study reported that the irregular washing of cups would enable H. pylori infection to increase (29), which may be a reason why the infection rate increased in the case of frequent tea drinking. Pickled foods contain high levels of carcinogens. Hence, the consumption of pickled foods has been listed among the main pathogenic factors of cancer, especially digestive tract cancers. Helicobacter pylori has been listed as a class I etiological factor of gastric cancer. It was found in this study that pickled food is also a risk factor of H. pylori infection, illustrating that cancer-inducing material in pickled food not only acts on the biochemistry mechanism of tissues and cells, but also indirectly affects the occurrence and development of gastric cancer, which is a risk factor of H. pylori infection.
5.4. The Relationship Between Helicobacter pylori Infection and General Social and Economic Conditions
It was found in the present study that the H. pylori infection rate was higher in a population with the education level of primary school or lower than in populations with the education level of junior middle school to special secondary school, as well as junior college or above. The H. pylori infection rate was lower in the population with a monthly income of more than 3000 Yuan than in a population with a monthly income of less than 3000 Yuan. In addition, the H. pylori infection rate was higher in migrant workers, fishermen, people without occupations, and general staff than in people with other occupations. These may be possibly associated with the lower income of these people, as well as the relatively poor living and health conditions. Furthermore, studies have shown that fatigue and non-regular rest are factors related to H. pylori infection (30). Among them, the H. pylori infection rate was significantly higher among fishermen than among other occupational groups, and this may be correlated with their residence in suburbs, as well as the relatively poor living conditions. Moreover, it may also be related to their frequent consumption of seafood, eating pickled foods, and more salt intake. In addition, it was also found in the present study that the H. pylori infection rate was higher in the married population than in the unmarried population, which may be related to the increase in household populations.
Helicobacter pylori infection has been reported to be related to the geographic area and socioeconomic status. It was found to be lower in developed countries than in developing countries (6). Furthermore, the H. pylori infection rate was generally lower than 30% in developed countries, while its positive infection rate might be as high as 50 - 70% in developing countries. For example, in the randomly selected 930 adults aged 35 - 74-years-old in four areas of Italy, the H. pylori infection rate was 45% (7). In the UK, the H. pylori infection rates were 37% in male employees and blood donors. In the United States, the H. pylori infection rate was approximately 30 - 40% in the general population. Some studies have pointed out that the poorer the economic status and the lower the degree of culture, the higher the H. pylori infection rate (8). The China H. pylori cooperative research group reported in a research of the epidemiology of H. pylori infection that the longer the years of education, the lower the H. pylori infection rate; the more the household populations, the higher the H. pylori infection rate; and the higher the income level, the lower the H. pylori infection rate.
5.5. Relationship Between Helicobacter pylori Infection and Personal Health Status
However, this study found that the H. pylori infection rate was higher in populations experiencing dyspeptic symptoms in recent years than in the normal population, which is consistent with the findings of many studies (31, 32). However, it remains controversial whether the eradication of H. pylori infection could improve the symptoms of functional dyspepsia (33, 34). In the future, the correlation between different digestive symptoms and H. pylori infection, as well as the improvement of digestive symptoms post-H. pylori eradication, may be further studied to clarify the correlation between H. pylori infection and gastrointestinal symptoms.
The results of this study revealed that the rate of infection was low among people who previously took an H. pylori test, as they have higher cognition of H. pylori, so that they have good dietary habits, reducing the chance of H. pylori infection. The present study found no significant differences in H. pylori infection rates between patients with diabetes and coronary heart disease and the general population, that is not consistent with previous reports (5, 35, 36). In the future, larger sample-sized, randomized, double-blinded, and more in-depth studies are required to explore the relationship between H. pylori infection and cardiovascular disease in the Binhai area. In addition, H. pylori strains should be isolated to perform a virulence analysis.
Research has shown that there is a correlation between H. pylori infection and the incidence of hypertension (37). The present study also found that the H. pylori infection rate was higher in hypertensive patients than in the general population, suggesting H. pylori infection is associated with hypertension. It was speculated that H. pylori infection may be involved in the pathophysiology of hypertension through some mechanisms; for example, H. pylori may affect endothelial function by regulating vascular active substances such as NO and endothelin-1. Helicobacter pylori infection is involved in oxidative stress, which leads to cellular apoptosis and the activation of tumor necrosis factor; these biological effects may lead to the occurrence of hypertension.
The results of the multivariate logistic regression analysis revealed that marital status and education level were important predictors of H. pylori infection. Gender and hypertension may be associated with H. pylori infection. H. pylori infection was negatively correlated with taking a previous Helicobacter pylori test. Since risk factors were more in the multivariate analysis and the number of H. pylori-positive cases and total sample size were relatively insufficient, the difference might be statistically insignificant.
5.6. Conclusions
In summary, the present study showed that the positive risk factors for H. pylori infection included male gender, smoking, drinking wine, eating out, eating seafood, eating pickled food, eating salt, drinking tea, keeping animals, poorer economic situation, lower income, marriage, and lower degree of culture while the negative risk factors were eating beans, eating uncooked garlic, eating vinegar, and eating spicy food. The prevalence of H. pylori infection and its related factors in the population of the Tianjin Binhai area were investigated for the first time in this survey. Our results revealed that H. pylori infection was related to education level and marital status. Furthermore, the relationships between health habits, diet, high blood pressure, gastrointestinal symptoms, and H. pylori infection needs to be further studied. By increasing the awareness of healthy diet among people, the promotion of personal hygiene habits, and the development of the people’s awareness of H. pylori infection, along with the development of the economy, we believe that the infection rate of H. pylori would show a downward trend year by year.