1. Background
Lactoferrin is one of the most important bioactive compounds of natural origin produced by fungi, bacteria, and viruses. It is found in large amounts in polymorphonuclear granules and various secretion fluids. It is a polypeptide chain of 689 amino acids containing both carboxylic acid and amine groups and is a spherical, multifunctional, and 80 kDa globular glycoprotein containing 700 amino acids from the transferrin family. Depending on the iron capacity, lactoferrin has various forms, including apo-, mono-, and holo- lactoferrin. Lactoferrin is known as one of the components of the innate immune system, which in addition to antibacterial, antifungal, anticancer, antiviral, and antioxidant properties, enhances the immune system and bone formation. The therapeutic role of lactoferrin in hepatic amebiasis has also been shown in a hamster model (1, 2). However, the antiparasitic activity of lactoferrin, due to its varied functions in different parasites, is still under discussion.
The in vitro and animal studies have indicated the protective effect of lactoferrin against intestinal microorganisms (1, 2). It has been displayed that lactoferrin protects children from gastroenteritis by preventing the connection of enteropathogens to the gut epithelium (1, 2). Further studies on the practical application of lactoferrin, as a major contributor to inflammation caused by infections, are necessary (3, 4). According to some investigations, the antimicrobial activity of multifunctional lactoferrin results from the function of lactoferrin cationic peptides against Gram-negative bacteria. Lactoferrin peptides have been proposed to be a suitable alternative to metronidazole with highly toxic effects (5-8).
Leishmaniasis, the most important parasitic zoonotic disease, has always been a major health problem in human societies, especially in endemic areas. Various factors, such as species, the amount and the entry site of the parasite, immune system status, and host genetics, are effective in the development and absence of this disease. Meglumine antimoniate and sodium stibogluconate are two drugs known as the first-line treatment of cutaneous leishmaniasis. The dangerous toxicity and side effects of common drugs, failure of some patients to respond to medications, relapse of the disease, and the simultaneous incidence of AIDS and leishmaniasis in patients have caused serious complications in the treatment process (9-11). Numerous studies have been conducted on various types of herbal, chemical, and nanoparticle medications to find a suitable and safe alternative medicine to common standard drugs and research in this area is still ongoing. On the other hand, there are two different roles for lactoferrin, including an antimicrobial role and a supporting role for some microorganisms (9, 10).
2. Objectives
Therefore, since there is no comprehensive study concerning the effectiveness of lactoferrin in cutaneous leishmaniasis in culture media, the present study was carried out to evaluate the effect of cytotoxic and apoptosis induction by lactoferrin on two forms of Leishmania major.
3. Methods
3.1. Preparation of Lactoferrin and Glucantime
First, 10 mg of the pure powder of lactoferrin was purchased from Sigma-Aldrich (USA) obtained from bovine milk as a globular glycoprotein with a molecular mass of about 80 kDa. Subsequently, a stock solution was prepared with distilled water to attain a desired consecrated solution by dilution. Glucantime was also purchased from Sigma-Aldrich and used as a standard control drug. Each test was repeated three times.
3.2. Leishmania major Culture
The Iranian standard strain of L. major (MRHO/IR/75/ER) was acquired from the Tehran University of Medical Sciences, Tehran, Iran. The category output of this parasite, which was purchased from Gibco (USA), was performed in RPMI 1640 media. Fetal bovine serum (10%; Sigma-Aldrich) was added to the media and mixed with antibiotics (100 unit/mL of penicillin and 100 µg/mL of streptomycin; Gibco). The culture was then kept in an 18 - 24ºC incubator and examined using an inverted microscope. Finally, a cell passage was conducted every 72 hours (12, 13).
3.3. Promastigote Growth Inhibition Test
To calculate the number of L. major promastigotes, similar aliquots of promastigotes) 106 cells/mL) were combined with various concentrations of lactoferrin, including 2.5, 5, 10, 20, 40, and 80 µg/mL, and incubated at 24, 48, and 72 h intervals. The temperature of the incubator was kept constant at 18 - 24ºC, and the test plates were examined using a microscope daily. One well containing promastigote and the medium was selected as a negative control, while another well containing promastigote, glucantime (20 µg/mL), and the medium was chosen as a positive control (12, 13).
3.4. MTT Assay of Anti-Leishmanial Activity of Lactoferrin Against Promastigotes
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay was employed to assess the anti-leishmanial activity of lactoferrin. For this purpose, some aliquots containing 106 logarithmic phase promastigotes were admixed with various concentrations of drugs in 96-well plates under completely sterile conditions. After filling the edge wells with PBS to decrease evaporation, the plates were incubated at 18 - 24ºC for 24, 48, and 72 h. Then, the medium-containing wells in each plate and the wells containing both medium and glucantime were selected as negative and positive controls, respectively. Following incubation, all the plates were centrifuged, and the supernatants were elutriated. The plates were anew incubated for 4 h at the same temperatures. Finally, formazan crystals were dissolved by the addition of dimethyl sulfoxide (DMSO) to the wells and mixed in the dark on a shaker for 10 - 15 min and examined at 490 nm (12).
3.5. MTT Assay to Assess Lactoferrin Toxicity to Macrophages
BALB/c peritoneal macrophages, the J774A.1 cell line, supplied by the Tehran University of Medical Sciences, were cultured at 105 cells per well in a plate before adding to given lactoferrin concentrations. To prevent evaporation in wells containing cells, the edge wells were filled with PBS. Then, 50 µL MTT solution (2 mg/mL in PBS) was added to the wells after 24, 48, and 72 hours, followed by incubation at 37ºC for 4 other hours. Next, the supernatants were removed and DMSO was added to all wells and read at the wavelength of 490 nm. To measure relative absorption, the optical density was calibrated versus the background of absorption in a well containing medium and macrophages, as the control (13).
3.6. Amastigote Growth Inhibition Test
In addition to macrophages and different doses of lactoferrin, promastigotes (106 cells) in the static phase were transferred to a chamber slide of eight wells. However, promastigotes in the stationary phase were added to each well at a parasite: macrophage ratio of 10:1 to contaminate macrophages. The chamber loaded just with macrophages was assigned as negative and the one loaded with both medium and glucantime (20 µg/mL) was assigned as the positive control. All the plates were then incubated at 37ºC for 24, 48, and 72 hours. The parasites that were not introduced to macrophages were gone from the environment by washing with PBS or culture media. The arranged smears were then made with methanol and discolored with Giemsa. Amastigotes in macrophages were counted by averaging the number of amastigotes in 100 macrophages (12, 13).
3.7. Flow Cytometry for Apoptosis Detection
Staining with annexin V and propidium iodide (PI) was performed to differentiate necrotic and apoptotic promastigotes from amastigote-infected macrophages previously exposed to different doses of lactoferrin. In this light, the cells exposed to different doses of the drug and the control cells exposed to PBS were centrifuged for 10 min at 1400 × g and then washed. As per the kit manual, annexin V and PI solutions were aggregated to sediment cells, and then the suspension was incubated in a dark place at room temperature for 15 min using a FACSCaliber device) FACSCanto II, BD Bioscience, San Jose, California, USA). Finally, the color intensity of absorbed annexin V by the cells was determined, and the analysis of the results was carried out with CellQuest (Tree Star, San Carlos, California, USA) (12, 13).
3.8. Statistical Analysis
By using a one-way analysis of variance, the mean number of parasites for each drug and its relevant concentrations was compared. The t-test was utilized to find significant differences between the control and test groups. The analyses were conducted by SPSS V. 20 software. The differences were regarded as statistically significant if P < 0.05. Values were expressed as mean ± SD, and the results were obtained by averaging at least three separate experiments (12, 13).
4. Results
4.1. Evaluation of Lactoferrin Effect on Parasite Counts
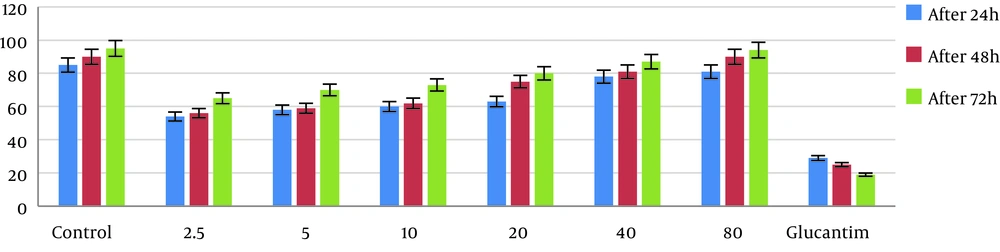
After 24, 48, and 72 hours of incubation in the presence of various doses of lactoferrin, the number of promastigotes at the 80 µg/mL concentration increased insignificantly and significantly over time compared to the control (P > 0.05) and glucantime (P < 0.05) groups, respectively. At the mentioned time intervals post-exposure, the parasite counts (mean ± SD) in the group exposed to 20 µg/mL concentration of glucantime were 0.7 ± 0.05, 0.68 ± 0.07, and 0.57 ± 0.03, while in the control group, they were 2.00 ± 0.02, 2.05 ± 0.03, and 2.09 ± 0.03, respectively. There were no significant differences between various concentrations of lactoferrin (P > 0.05; Table 1).
| Lactoferrin, µg/mL | Promastigotes (106) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 2.5 | 0.49 ± 0.03 | 0.85 ± 0.03 | 1.12 ± 0.02 |
| 5 | 0.52 ± 0.01 | 0.88 ± 0.03 | 1.14 ± 0.03 |
| 10 | 0.61 ± 0.03 | 0.92 ± 0.03 | 1.21± 0.02 |
| 20 | 0.7 ± 0.02 | 1.09 ± 0.04 | 1.25 ± 0.03 |
| 40 | 1.10 ± 0.03 | 1.20 ± 0.03 | 1.31 ± 0.02 |
| 80 | 1.65 ± 0.02 | 1.74 ± 0.04 | 1.87 ± 0.03 |
Number of Promastigotes After the Addition of Lactoferrina
4.2. Viability of Parasites by MTT Assay
Promastigotes exposed to the highest concentration of lactoferrin (80 µg/mL) after 72 h incubation showed 84% survival, which was not significantly different from the control test (P > 0.05). Also, no significant difference was found between various concentrations of lactoferrin (P > 0.05). The survival percentages of the parasites in the group receiving 20 µg/mL of glucantime were 24%, 20%, and 16%, but in the control group, they were 85%, 93%, and 97% after 24, 48, and 72 hours, respectively.
4.3. Evaluation of Toxicity to Macrophage Cells by MTT Assay
Using MTT, the toxicity of various concentrations of lactoferrin to macrophage cells was assessed. The results demonstrated the lower toxicity of lactoferrin (2.12%) at 80 µg/mL after 72 hours. These results were the average of at least three separate experiments.
4.4. Number of Amastigotes Inside Macrophage Cells in the Presence of Lactoferrin
The results of intracellular amastigotes showed that lactoferrin did not reduce the viability of amastigotes but increased the growth of amastigotes in J774.A1 macrophages at a concentration and dose-dependent manner. Differences between the 80 µg/mL experimental group and the control group were not significant (P > 0.05). However, no significant differences were detected between different concentrations of lactoferrin (P > 0.05). Parasite survival percentages in macrophages at 20 µg/mL concentration of glucantime after 24, 48, and 72 hours were 29%, 25%, and 19%, while those of the control group were 85%, 90%, and 95%, respectively (Figure 1).
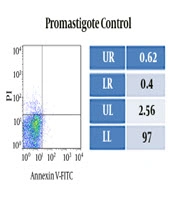
4.5. Flow Cytometry
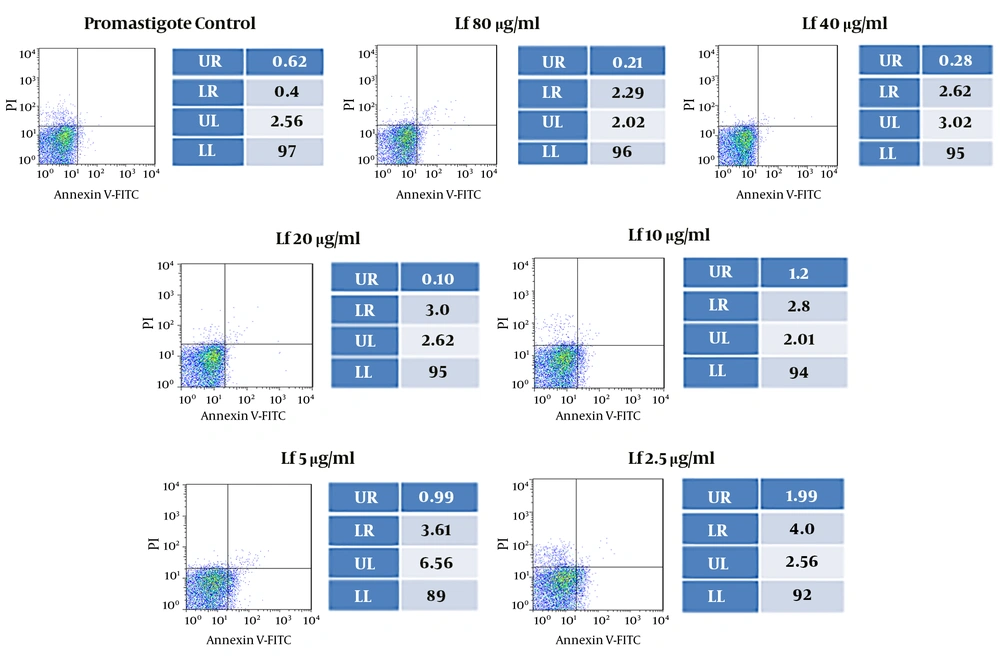
The programmed cell death of Leishmania promastigotes cultured in the control media was 1.02%, while that of amastigote-infected macrophages was 12.10% after 72 hours of culture. The results of both promastigotes and amastigote-infected macrophages were not statistically significantly different from the results of the control groups (P > 0.05). Flow cytometry investigation indicated that lactoferrin did not induce early and late apoptosis in the parasite. In promastigotes treated with 80, 40, 20, 10, 5, and 2.5 µg/mL of lactoferrin, the apoptosis rates were 2.5%, 2.9%, 3.1%, 4.0%, 4.6%, and 5.99%, whereas the apoptosis rates of infected amastigotes were 7.40%, 7.90%, 8.89%, 9.59%, 10.9%, and 10.87%, respectively, after 72 hours of exposure (Figures 2 and 3).
Flow cytometry analysis of promastigotes after enhancing various concentrations of lactoferrin for 72 hours and after labeling with annexin V and PI. Lower and upper right regions (LR) belong to early and late apoptotic cells (annexin positive) and the upper left region (UL) belongs to necrotic cells (PI-positive).
5. Discussion
Treatment is necessary to control a disease, especially in conditions that the source of the disease is the human; however, drug resistance gives rise to many limitations and difficulties in the treatment process. Lactoferrin is a key element in the mammalian immune system due to its iron diffusion in the mucosa. Indeed, it plays an important role in the host defense against infections and inflammation and it is indirectly involved in specific immune responses (14, 15). Lactoferrin has also been suggested to have synergistic effects with antibiotics and drugs and even with other proteins of the innate immune system. In a mouse model, this protein orally controls parasitic infection by killing the parasite or via the reconstruction of the intestinal anti-inflammatory environment. Researchers have exhibited that the lactoferrin-iron complex produces free oxygen radicals that damage to the membrane of red blood cells and parasites, and this stage results in the prevention of the parasite growth (4-6).
As one of the mammalian glycoproteins, lactoferrin adsorbs extracellular iron from mucosal surfaces such as colon levels, where amoeba causes infection. The amoeba cysteine proteinase destroys the holo-lactoferrin. Amoeba trophozoites are connected to lactoferrin through specific membrane lactoferrin-binding proteins, and Entamoeba histolytica requires iron for its metabolism. Killing the histolytic amoeba in the culture media by this protein confirms its therapeutic effect. In E. histolytica, cysteine protease breaks down lactoferrin. The anti-amoeba activity of lactoferrin depends on time and density. Trichomonas vaginalis, Toxoplasma and, E. histolytica express lactoferrin-binding proteins to use holo-lactoferrin, as an iron source, for the growth in culture media. Iron uptake and increased intracellular enzyme activity lead to host lactoferrin binding by T. vaginalis receptors (14-16). Leishmania chagasi uses the iron to bind to transferrin or lactoferrin. Promastigotes preferentially adsorb iron in the reduced or oxidized forms; hence, extracellular iron needs to be decreased before entering the cell. The iron absorption in Trypanosoma, in contrast to L. chagasi, requires a specific receptor. Accordingly, the presence of the specific receptor in parasite results in the application of various iron sources in the host environment.
The results displayed that the growth of Leishmania promastigotes increased after adding iron to the culture medium. Leishmania chagasi promastigotes obtain iron from free lactoferrin, hemin, and free transferrin. The iron or heme connected to lactoferrin leads to the normal growth of the parasite in culture media. Iron is absorbed from transferrin, lactoferrin, or heme, but the absorption of iron from lactoferrin is faster, which does not correlate with the growth stage of the parasite. In insects and mammals, the capacity for using various iron sources in L. chagasi elevates the ability of the organism to survive in various environments. Lactoferrin bonded with amphotericin B along with the LcfPGNP-AmB nanoparticle has anti-leishmaniasis effects, which decreases parasite burden in the spleen and increases immunity in the hamster. Iron is an essential nutrient for pathogen survival in the host cell (17-19). As a general strategy against microbes in mammals, the inhibitor complex system of iron has evolved to reduce the microbes access to iron. The pathogens in the respiratory, intestinal, urinary, and genital tracts, when exposed to the lack of iron on the mucosal surfaces, utilize ferric iron-binding lactoferrin, as an extracellular glycoprotein.
On the other hand, protozoans have developed varied mechanisms for obtaining iron from host holo-lactoferrin. Leishmania promastigotes use surface reductase at the time of ferric iron reduction to give access to the ferrous form. Previous studies have affirmed that the L. chagasi promastigote forms can use lactoferrin and transferrin iron for growth and metabolism (17-19). The findings have also suggested that the promastigote form has specific locations for connection between lactoferrin and transferrin. Lactoferrin connection, as an antiseptic and inflammatory protein, is independent of the presence or absence of iron-containing proteins and is not inhibited by transferrin. Besides, it is independent of the growth phase of the microorganism. No information is available on the iron source for the proliferation of amastigotes inside macrophages. Therefore, various microbes apply different mechanisms for using lactoferrin iron and their survival in the host environment. Leishmania (chagasi) or its species utilize iron for binding to transferrin, lactoferrin, or other chelates. Promastigotes adsorb iron in a reduced rather than an oxidized form because promastigotes present an NADPH-dependent iron reductase activity, and Leishmania is a parasite-associated or -secreted reductase that diminishes ferric to ferrous iron, and this ability assist the parasite to internalize iron.
These behaviors could justify the capability of the parasite in using iron from multiple sources in different host environments (17-19). Trypanosoma cruzi requires both heme and non-heme iron for better growth in vitro. The increased or decreased iron levels affect the growth of the parasite; thus, only if necessary, the parasite obtains the required iron for the growth from the intracellular iron. Therefore, the separation of iron from intracellular deposits, at the time of iron deficiency, elevates the pathogenesis of intracellular parasites. Intracellular effects of increased or decreased levels of iron are associated with the parasite growth rate and pathogenicity (10, 14). A former study has investigated the pathological and protective effects of lactoferrin on mouse Leishmania and suggested that oral lactoferrin, for 10 days, is appropriate for the treatment of L. major.
The results of the mentioned study showed a higher titer of IgG in the group treated with lactoferrin, and the pathological results demonstrated the therapeutic effect of the lactoferrin-treated group in vivo, through modification of the phagocytic capacity of macrophages and neutrophils, in the recovery from infection. L. major and L. tropica can affect the serum levels of copper, zinc, and iron, thereby leading to the decreased levels of zinc and iron and increased level of copper in the serum of patients (20).
Lactoferrin has two major effects on some intestinal pathogens, i.e., prevention of the growth and inhibition of the expression of surface pathogenic factors. By depriving the pathogen of iron or plasma membrane degradation through high cationic charge, lactoferrin exerts its antimicrobial effect. The multifunctionality of lactoferrin is due to positive charging and its distribution in different tissues. Its antimicrobial activity could be attributed to two major mechanisms: (1) iron absorption at the site of infection to prevent the microorganism from nutrition and inhibition of parasite growth and (2) direct interaction with microorganisms. The reaction of positive amino acids in lactoferrin with anionic molecules in some microorganisms can cause the lysis of the cell (21, 22).
Lopez-Soto et al. examined the anti-amoeba effect of apo-lactoferrin, a protein in milk, and found that this protein ruptured the membrane and destroyed the parasite in the culture medium owing to binding to the membrane lipid of the trophozoite (6). Dzietko et al. observed that holo-lactoferrin may bind to intracellular parasites. Lactoferrin can prevent the further growth of the parasite inside the cells but it could not prevent the parasite from entry into the cell (23). Ikada et al. (24) displayed that apo-lactoferrin greatly suppressed Babesia caballi, but other types of lactoferrin had no impact on the parasite, and in the case of B. equi, none of the types of lactoferrin could affect the parasite. Therefore, the effect of lactoferrin on these blood parasites is related to bonding to iron (III).
Most studies have been conducted on the effect of lactoferrin in the presence or absence of iron (III) in vitro (19, 24). In this study, by calculating the number and percentage of live parasites using the MTT method, concerning the concentration and time, we observed that the number of parasites enhanced with both concentration (80 µg/mL) and time (72 h), and there was no significant difference between the treatment and control groups. Importantly, we did not have a decrease in the number of parasites. The results of this study did not show any apoptosis effects on both forms of L. major.
5.1. Conclusions
The results of the previous studies revealed that in some parasites, lactoferrin has a stimulant effect and in some cases, it inhibits growth. This research, for the first time, investigated the effect of lactoferrin on L. major in vitro, which conformed and contrasted to previous findings. We suggest that other types of lactoferrin and various lactoferrin peptides be used to treat leishmaniasis and that major causes of growth or inhibition of the Leishmania parasite by lactoferrin be studied in further studies.
5.2. Ethics Statement
This study was reviewed by the Ethics Committee of the Iran University of Medical Sciences following the Helsinki Declaration and approved with code no.: IR.IUMS.REC.FMD. 96-02-30-31136.
5.3. Human and Animal Rights
No animals/humans were used for studies that are the basis of this research.