1. Background
Cockroaches are one of the predominant insects in residential environments including health care settings and hospitals. They have been tremendously fruitful in misusing the spaces within human habitation. Cockroaches are recognized for survival, transmission, and spread of hazardous microbial pathogens (1, 2). Two of the most imperative and mutual cockroach species originated in Iranian hospitals are American (Periplaneta americana) and German cockroaches (Blattella germanica). Periplaneta americana is larger and typically has a glossy reddish-brown color (1, 2). Both of them are acknowledged as the reservoirs of pathogenic bacteria, predominantly Staphylococcus aureus (3, 4).
Staphylococcus aureus usually exists in the nose, respiratory tract, and the skin (5). It is accountable for the outbreaks of nosocomial and community-acquired infections including wound and burn infections, urinary tract infections (UTIs), respiratory tract infections (RTIs), blood and soft tissues infections, food-borne diseases, and food poisoning (5). Staphylococcus aureus is habitually resistant toward numerous types of antibiotic agents (6-9). Some of them are resistant toward methicillin antibiotics, which are named as methicillin-resistant S. aureus (MRSA). Methicillin-resistant S. aureus bacteria have a boosted irrefutable standing owing to their substantial resistance toward various types of antibiotics, leading to their higher pathogenicity (6-9).
Staphylococcal chromosomal cassette mec (SCCmec) is a genetic division of the MRSA bacteria accompanying with the mecA gene and is responsible for virulent characteristics (10). It is predominantly originated in MRSA bacteria of the hospital environment. It is characteristically grouped into types I, II, III, IV, and V rendering to ccr and mec alleles (10). The SCCmec type IV is further grouped into a, b, c, and d types (10). The pathogenicity of MRSA bacteria correspondingly relies on abundant surface antigens and extracellular proteins. MRSA strains are mainly produce leukocidal toxins, which suggests that Panton-Valentine leucocidin (PVL) is one of the most imperative virulence factors with substantial contribution to the pathogenicity of diseases caused by MRSA bacteria (11).
2. Objectives
The current survey was performed to measure the frequency of SCCmec types and the PVL gene among MRSA bacteria recovered from external washing samples of P. americana and B. germanica hospital cockroaches.
3. Methods
3.1. Bacteria and Further Identification
We recovered 36 MRSA isolates from external washing samples of hospital cockroaches (12). Cockroaches were obtained from private hospitals of Tehran Province, Iran, using standard traps (13). After immobilization by freezing (0°C for 5 min), the species of cockroaches were identified under a dissecting microscope according to the method by Harwood and James (14). Methicillin-resistant S. aureus bacteria were recovered from P. americana and B. germanica hospital cockroaches based on a previously described procedure (13). Methicillin-resistant S. aureus bacteria were confirmed using cefoxitin (30 µg) and oxacillin (1 µg) susceptibility tests. Examinations were done based on the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (15).
3.2. DNA Extraction
Methicillin-resistant S. aureus bacteria cultured on the tryptic soy broth (Merck, Germany) medium were analyzed by the DNA extraction kit (Thermo Fisher Scientific, Germany) following the manufacturer’s guidelines. Purity, quality, and quantity of DNA were assessed using the previously described procedures (6, 12).
3.3. PCR-Based Detection of SCCmec Types and PVL Gene
Table 1 exemplifies the PCR circumstances applied for the identification of SCCmec types and the PVL gene (16, 17). A programmable DNA thermo-cycler (Eppendorf Mastercycler 5330, Germany) was used for this purpose. Amplified samples were apprised by electrophoresis based on a previously described technique (16, 17).
| Target Gene | Primer Sequence (5’ - 3’) | PCR Product, bp | PCR Program, s | PCR Volume (50 µL) |
|---|---|---|---|---|
| SCCmec I | F: GCTTTAAAGAGTGTCGTTACAGG | 613 | 1 cycle: 93°C, 7 min. 10 cycles: 93°C C, 55 s; 64 °C, 50 s; 72°C, 2 min. 25 cycles: 94°C, 45 s; 55°C C, 45 s; °C, 2 min. 1 cycle: 72°C, 10 min | 5 µL PCR buffer 10×; 2 mM MgCl2; 150 µM dNTP (fermentas); 0.75 µM of each primers F & R; 1.5 U Taq DNA polymerase (fermentas) ; 3 µL DNA template |
| R: GTTCTCTCATAGTATGACGTCC | ||||
| SCCmec II | F: CGTTGAAGATGATGAAGCG | 398 | ||
| R: CGAAATCAATGGTTAATGGACC | ||||
| SCCmec III | F: CCATATTGTGTACGATGCG | 280 | ||
| R: CCTTAGTTGTCGTAACAGATCG | ||||
| SCCmec IVa | F: GCCTTATTCGAAGAAACCG | 776 | ||
| R: CTACTCTTCTGAAAAGCGTCG | ||||
| SCCmec IVb | F: TCTGGAATTACTTCAGCTGC | 493 | ||
| R: AAACAATATTGCTCTCCCTC | ||||
| SCCmec IVc | F: ACAATATTTGTATTATCGGAGAGC | 200 | ||
| R: TTGGTATGAGGTATTGCTGG | ||||
| SCCmec IVd | F: CTCAAAATACGGACCCCAATACA | 881 | ||
| R: TGCTCCAGTAATTGCTAAAG | ||||
| SCCmec V | F: GAACATTGTTACTTAAATGAGCG | 325 | ||
| R: TGAAAGTTGTACCCTTGACACC | ||||
| PVL | F: ATCATTAGGTAAAATGTCTGGACATGATCCA | 433 | 1 cycle: 94°C, 5 min. 30 cycle: 94°C, 30 s; 55 °C, 30 s; 72°C, 30 s. 1 cycle: 72°C, 5 min | 5 µL PCR buffer 10×; 2 mM MgCl2; 150 µM dNTP (fermentas); 0.75 µM of each primers F & R; 1.5 U Taq DNA polymerase (fermentas) ; 3 µL DNA template |
| R: GCATCAASTGTATTGGACATGATCCA |
3.4. Statistical Analysis
Statistical analysis was done using SPSS 21.0 Numerical software (SPSS Inc., Chicago, IL, USA). The chi-square test and Fisher’s exact two-tailed test were applied to assess any significant relationship between the frequency of SCCmec types and the PVL gene among MRSA bacteria. A P value of < 0.05 was considered the significance level.
4. Results
4.1. SCCmec Types
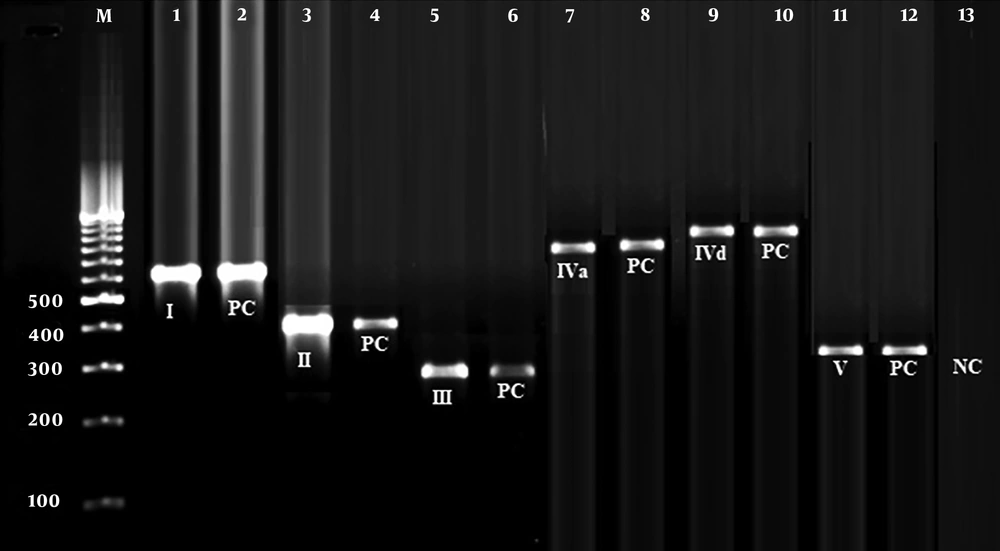
Figure 1 signifies the results of the gel electrophoresis of the SCCmec types found in MRSA bacteria. Table 2 shows the frequency of SCCmec types and the PVL gene among MRSA bacteria. We observed that SCCmec III (44.44%), I (27.77%), and II (16.66%) were the most frequent types among MRSA bacteria. There were no SCCmec types IVb and IVc among MRSA bacteria. The frequency of SCCmec type I was 28.57% and 25%, type II was 17.85% and 12.50%, and type IIIs was 42.85% and 50% among P. americana and B. germanica hospital cockroaches, respectively. Statistical significant difference was found between kind of samples and frequency of SCCmec types (P < 0.05).
| Samples (Number of Positive for MRSA) | Number of Positive Samples for Each SCCmec Type (%) | Number of Positive Samples for PVL Gene (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||||
| a | b | c | d | ||||||
| P. americana (n = 28) | 8 (28.57) | 5 (17.85) | 12 (42.85) | 1 (3.57) | - | - | 1 (3.57) | 1 (3.57) | 10 (35.71) |
| B. germanica (n = 8) | 2 (25) | 1 (12.50) | 4 (50) | - | - | - | - | 1 (12.50) | 2 (25) |
| Total (n = 36) | 10 (27.77) | 6 (16.66) | 16 (44.44) | 1 (2.77) | - | - | 1 (2.77) | 2 (5.55) | 12 (33.33) |
aValues are expressed as No. (%).
4.2. PVL Gene Frequency
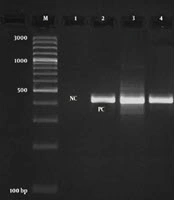
Figure 2 signifies the results of the gel electrophoresis of the PVL gene in MRSA bacteria. Twelve out of 36 (33.33%) MRSA bacteria harbored the PVL gene. The frequency of the PVL gene was 35.71% and 25% among MRSA bacteria isolated from P. americana and B. germanica hospital cockroaches, respectively. Statistical significant difference was found between d of samples and frequency of the PVL gene (P < 0.05).
5. Discussion
Cockroaches are regarded as the sources of diverse types of antibiotic-resistant bacteria. They correspondingly can be the vectors for the transmission of numerous diseases into human inhabitants, predominantly in public places such as hospitals (18). The results of the current survey signified that MRSA bacteria recovered from hospital cockroach samples harbored the PVL gene and various types of SCCmec units. Methicillin-resistant S. aureus bacteria harbored a high frequency of SCCmec types III, I, and II. Methicillin-resistant S. aureus is usually sub-categorized into healthcare-associated MRSA (HA-MRSA) and community-associated MRSA (CA-MRSA). Epidemiological surveys show that HA-MRSA bacteria harbor SCCmec I, II, or III while CA-MRSA bacteria harbor SCCmec types IV or V (19). Furthermore, HA-MRSA bacteria have a lower frequency of the PVL gene than CA-MRSA bacteria (19). Consequently, the majority of MRSA bacteria recovered from external washing samples of hospital cockroaches were HA-MRSA.
The possible reason for the higher frequency of HA-MRSA bacteria in the examined samples is that external washing samples of hospital cockroaches contained MRSA bacteria of the hospital environment, which were carried owing to the contact of the exterior parts of the cockroach’s bodies with the sources of infections existed in the hospitals. The previously available data convey that the majority of PVL-positive S. aureus bacteria are related to soft tissue and skin infections (20). Consequently, PVL-positive MRSA bacteria recovered from hospital cockroaches may originate from the cases of soft tissue infections in hospitals. Methicillin-resistant S. aureus has been presented as one of the most predominant pathogenic bacteria recovered from B. germanica and P. americana cockroaches (3, 4, 21-23). Nevertheless, the isolation of the SCCmec types and the PVL gene among MRSA bacteria recovered from hospital cockroaches was not scrutinized beforehand.
Borbon-Esquer et al. (24) conveyed that of 102 MRSA bacteria recovered from hospitalized children in Mexico, 97 (95%) harbored SCCmec type II, 5 (5%) harbored SCCmec type Iva, and all (100%) of them were PVL-negative. Momtaz and Hafezi (25) conveyed that SCCmec type III (24.52%) had the highest frequency among MRSA bacteria recovered from clinical infections. They signified that the frequency of the PVL gene was 40.90%. Higher frequency of SCCmec types I, II, and III was also reported from India (26), Saudi Arabia (27), and Brazil (28). Goudarzi et al. (29) indicated that diverse SCCmec types including SCCmec type III (38.9%), II (31.1%), IV (28.9%), and I (1.1%) were obtained from MRSA bacteria recovered from the cases of UTIs in Iran.
We showed that only 33.33% of the MRSA bacteria harbored the PVL gene. The PVL gene is one of the main exotoxins of MRSA. The occurrence of the PVL gene in MRSA bacteria recovered from clinical infections was also reported beforehand (30, 31). The occurrence of the PVL gene among SCCmec types I, II, and III bacteria was also conveyed by Lima et al. (32) and Glikman et al. (33). Nevertheless, PVL-positive isolates were not discovered in SCCmec types I, II, and III bacteria in previous research (34, 35). Consequently, it can be concluded that the existence of the PVL gene is not an explanatory factor for CA-MRSA, as it may be existed in HA-MRSA or maybe absent from CA-MRSA.
It is known that SCCmec types I, II, and III are present in HA-MRSA strains and SCCmec types IV and V are present in CA-MRSA strains. The studied cockroaches could move freely inside and outside the hospital environment. Thus, they could carry both HA-MRSA (from the hospital environment) and CA-MRSA (from outside the hospital environment) strains as reported in our survey. Thus, it is not surprising that both CA-MRSA and HA-MRSA strains were found in the studied samples. Additionally, MRSA bacteria isolated from B. germanica and P. americana cockroaches harbored various SCCmec types. This may be probably because of differences in the type of feeding, lifestyle, and crossing paths and diverse living locations of the two cockroach species inside or outside the hospital environment.
5.1. Conclusions
The current survey is an initial description of the identification of SCCmec types and the PVL gene among MRSA bacteria recovered from external washing samples of hospital cockroaches. High frequency of SCCmec types I, II, and III and comparatively low frequency of the PVL gene characterize the occurrence of HA-MRSA bacteria in B. germanica and P. americana hospital cockroaches. This finding discloses an imperative public health hazard concerning the attendance of HA-MRSA bacteria in hospital cockroaches. The results showed that B. germanica and P. americana hospital cockroaches are the reservoirs of MRSA bacteria in the hospital environment. Further surveys are mandatory to gain supplementary information about the epidemiological share of hospital cockroaches in the survival and transmission of MRSA bacteria.