1. Background
Nosocomial infections constitute the major reason for death among inpatients. These infections, which are often acquired as a result of urinary tract infection (UTI), surgical wounds and lower respiratory tract infections, might be endogenous or cross-infection (1). Urinary tract infections (pyelonephritis and cystitis) are considered to be the second major hospital infections in humans (1, 2). Though this infection is considered to be an easily curable disease, an estimated 150 - 250 million patients of UTI are identified yearly worldwide, accounting for > 7 million doctor office visits in the United States (3). Moreover, 70% - 95% of the UTI occurs by urinary pathogenic Escherichia coli (UPEC) (4). The host’s fecal flora is the major usual quick origin of E. coli infections (5, 6).
The E. coli virulence factors (VFs) consist of protectins such as serum resistance (traT) (7), associated islands (PAIs) (8) and Tir-containing protein of E. coli (tcpc) (9); iron uptake such as aerobactin (aer); cytotoxic necrotizing factor 1 (cnf1) and hemolysin A (hlyA); adhesins such as type 1 fimbriae (fimH), A fimbriae (afa), S fimbriae (sfa), and P fimbriae (papG) (10, 11). Uropathogenic E. coli produces a number of VFs, which protect these strains in the face of very effective host protection and make it possible for them to colonize in the urinary tract (11). Uropathogenic E. coli strains display a wide genomic variety, due to having particular VFs located on mobile genetic elements called PAIs, that are common in the horizontal transition of VFs (12). Aer gene encodes Aerobactin (siderophore) and has newly been recognized as a VF in UPEC (13, 14). Other VFs such as serum resistance capacity, due to TraT protein (the external membrane protein) encoded by traT genes, have significant roles in the progression of UTI (15).
The treatment of UTI caused by UPEC often needs antibiotic therapy. However, currently, there has been an increase in the rate of antibiotic-resistant E. coli strains, and UTI are considered an important health concern (16, 17). The emergence of antimicrobial resistance, especially extended-spectrum beta-lactamases (ESBLs) and mainly multidrug-resistant (MDR) in UPEC strains rise the very important danger to worldwide public health (18, 19). Therefore, it is necessary to determine the factors related to the pathogenicity of bacteria in each region to control the infection and help eradicate the pathogen. Also, examining the antibiotic resistance pattern of bacteria in each region will allow physicians to prevent the development of antibiotic resistance by selecting the appropriate antibiotic.
2. Objectives
This study aimed to explore antimicrobial susceptibility and identify aer, traT, and PAI genes in E. coli isolates collected from fecal and UTI specimens and determine the relationship between PAI, traT, and aer genes in both populations studied in a center in Iran by multiplex polymerase chain reaction (PCR) assay.
3. Methods
3.1. Bacterial Isolates
Uropathogenic E. coli isolates (n = 75) and commensal fecal without UTI and diarrhea (n = 75) were collected from the patients of educational hospitals, Shahrekord Center, Iran, 2017. A number of 75 E. coli isolates from the urine of patients with UTI containing significant counts (≥ 105 CFU/mL) were included in this study. Escherichia coli isolates were identified by Gram staining, culture on Eosin methylene blue (EMB) agar, MacConkey agar, and Blood agar (Merck Co., Germany), and biochemical tests (IMViC tests).
3.2. Antimicrobial Susceptibility Testing
Antibiotic susceptibility patterns for 14 antibiotics were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) protocols by the disk-diffusion technique (20). The antibiotic susceptibility of the bacteria was determined by the disc diffusion method (Kirby-Bauer method) for 14 antibiotics. After incubation, the diameter of the inhibition zone was measured and interpreted according to the standard antibiogram. The following antibiotics (Mast, Co) were included in this study: ampicillin (100 mg), imipenem (10 mg), piperacillin (10 mg), amikacin (30 mg), cotrimoxazole (25 mg), ciprofloxacin (5 mg), ceftazidime (30 mg), nitrofurantoin (300 mg), gentamicin (10 mg), ceftriaxone (30 mg), nalidixic acid (30 mg), cefalotin (5 mg), cefepime (30 mg), and tazocin (30 mg). The E. coli ATCC 25922 was used as the control strain.
3.3. DNA Extraction and Multiplex PCR
DNA extraction was done by the boiling method. To extract DNA from the isolates, they were first cultured on the EMB medium. For boiling, 3 - 5 colonies were inoculated into a microtube containing 1,000 μL of Tris buffer. The microtubes were then placed on a hot plate for 15 minutes at 95°C. Next, they were centrifuged at 14,000 rpm for 10 minutes. Finally, the supernatant containing DNA was transferred to another microtube (21). The purity of the DNA was measured by a NanoDrop™ spectrophotometer. DNA samples by an OD260/OD280 ratio of ≥ 1.8 were used for the next phase of the study. Multiplex PCR was performed for aer (22), traT, and PAI (23). The sequences of primers used for this study are listed in Table 1. The multiplex PCR was optimized according to the following experimental conditions: initial denaturation at 95°C for five minutes, 35 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 40 seconds, followed by a final extension step at 72°C for five minutes and holding a one-time cycle at 4°C for 5 minutes. In this test, E. coli ATCC 700928 was the positive control and 16S rRNA gene was the internal control (24).
| Gen Type | Primers Sequences (5’ - 3’) | Size |
|---|---|---|
| aer | F: TACCGGATTGTCATATGCAGACCGT | 602 |
| R: AATATCTTCCTCCAGTCCGGAGAAG | ||
| traT | F: GGTGTGGTGCGATGAGCACAG | 290 |
| R: CACGGTTCAGCCATCCCTGAG | ||
| PAI | F: GGACATCCTGTTACAGCGCGCA | 930 |
| R: TCGCCACCAATCACAGCCGAAC | ||
| 16SrRNA | F: AGGCCTTCGGGTTGTAAAGT | 420 |
| R: ACCTCCAAGTCGACATCGTT |
3.4. Statistical Analysis
The Statistical Package for Social Sciences (SPSS) was used for data analysis. The significance level was set at 0.05. Comparisons of proportions were tested using chi-square or Fisher’s exact test (two-tailed), as appropriate.
4. Results
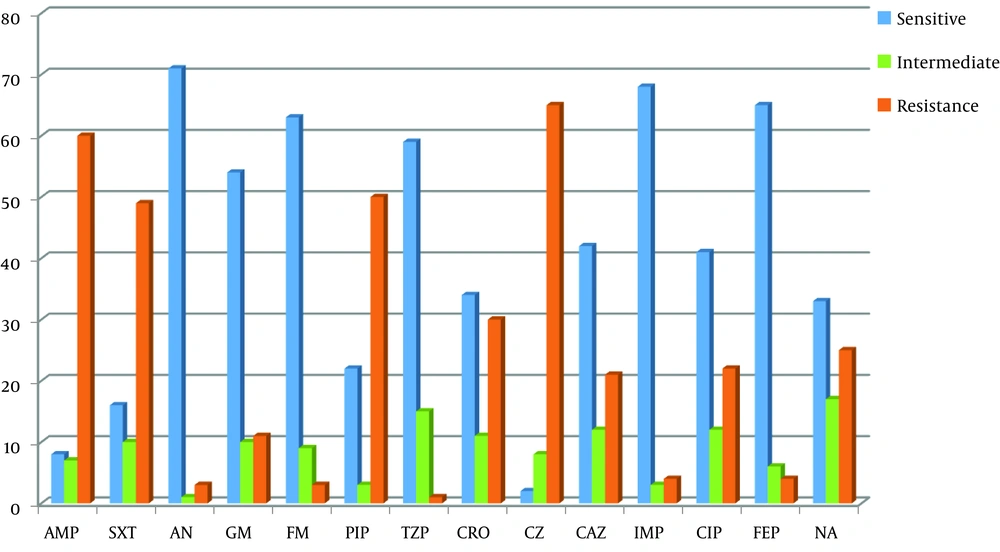
In this study, 41.33% of the patients were male, while 58.67% were female. Among the UPEC isolates, the highest antibiotic resistance was reported on cefazolin, ampicillin, and cotrimoxazole antibiotics. Most isolates were susceptible to amikacin, imipenem, cefepime, tazocin, and nitrofurantoin. The antibiotic resistance pattern for 14 antibiotics on 75 UTI isolates is presented in Figure 1.
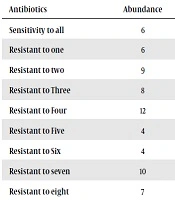
No antibiotic resistance to more than 10 antibiotics was observed in this study (Table 2). Multidrug resistance patterns were determined based on resistance to three or more different classes of antibiotics. It was found to be 62.67%.
| Antibiotics | Abundance | Frequency (%) |
|---|---|---|
| Sensitivity to all | 6 | 8 |
| Resistant to one | 6 | 8 |
| Resistant to two | 9 | 12 |
| Resistant to Three | 8 | 10.66 |
| Resistant to Four | 12 | 16 |
| Resistant to Five | 4 | 5.33 |
| Resistant to Six | 4 | 5.33 |
| Resistant to seven | 10 | 13.33 |
| Resistant to eight | 7 | 9.33 |
| Resistant to Nine | 5 | 6.66 |
| Resistant to ten | 2 | 2.66 |
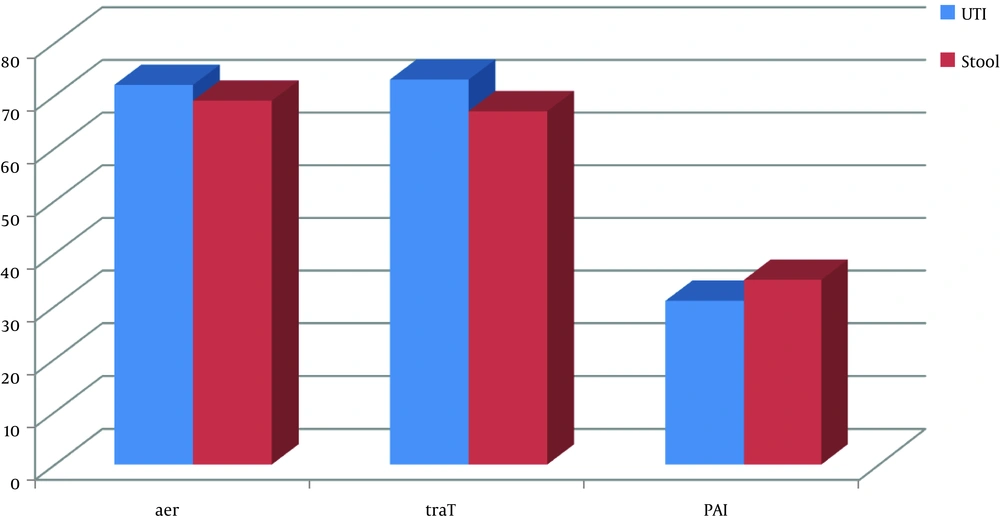
In this study, in spite of the abundance of the aer, traT, and PAI genes studied in both UPEC isolates and commensal fecal without UTI and diarrhea isolates populations (Figure 2), there was no correlation between the genes in the two populations based on P values (P > 0.05) (Table 3).
| Gene | Uropathogenic | Commensal | Total | P Value |
|---|---|---|---|---|
| Aer | 96 | 92 | 94 | 0.494 |
| traT | 97.3 | 89.3 | 93.3 | 0.05 |
| PAI | 41.3 | 46.7 | 44 | 0.511 |
The frequencies of aer gene in the E. coli isolated from urine and fecal samples were 96% (n = 72) and 92% (n = 69), respectively. Also, the total frequency of this gene was 94% (n = 141). The frequencies of traT gene in the E. coli isolated from urine and fecal samples respectively were 97.3% (n = 73) and 89.3% (n = 67), and totally it was 93.3% (n = 140). Furthermore, the frequencies of PAI gene in the E. coli isolated from urine and fecal samples were 41.33% (n = 31) and 46.67% (n = 35) in that order. The total frequency of PAI gene in both groups it was 44% (n = 66) (Tables 4 and 5).
| Number | Only PAI | Only traT | Only Aer | traT, PAI | aer, PAI | Aer, traT | traT, PAI, Aer |
|---|---|---|---|---|---|---|---|
| UTI | 0 | 1 | 1 | 2 | 1 | 42 | 28 |
| Stool | 1 | 3 | 5 | 2 | 2 | 32 | 30 |
| Sum | 1 | 4 | 6 | 4 | 3 | 74 | 58 |
| Aer | traT | PAI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P Value | No | Yes | P Value | No | Yes | P Value | |
| UTI | 72 | 3 | 0.494 | 73 | 2 | 0.05 | 31 | 44 | 0.511 |
| Stool | 69 | 6 | 67 | 8 | 35 | 40 | |||
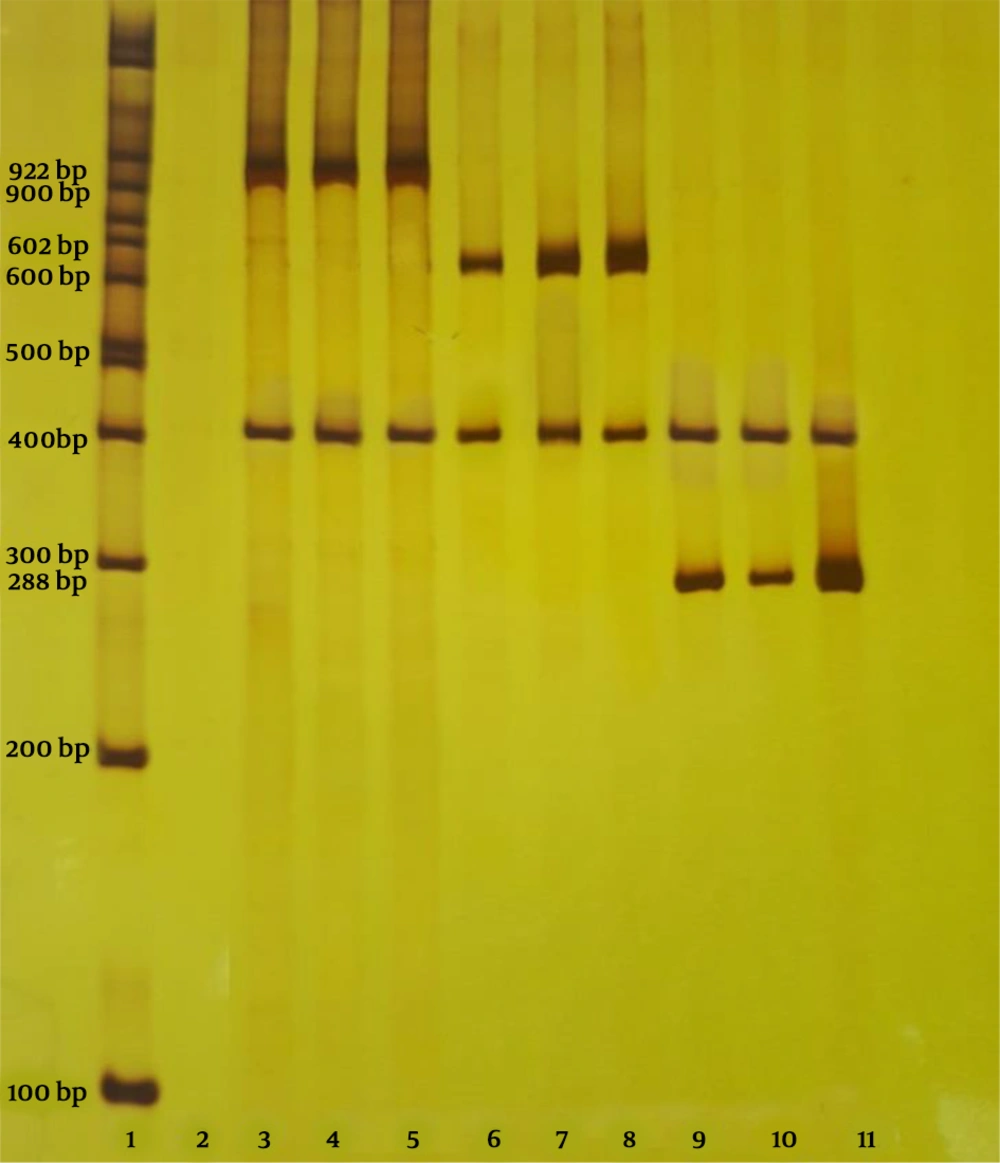
Regarding the study of genes in the E. coli isolated from urine of patients with UTI and feces from healthy individuals, despite the high frequency of PAI, traT, and aer genes, it cannot be concluded that the relationship between genes is the same as in both groups, according to the tables. The high frequency of these genes can be attributed to the bacteria’s need for these genes for growth and combined with some other virulence factors to cause UTI. The electrophoresis technique was performed on the polyacrylamide gel after completing the PCR steps. After electrophoresis, the polyacrylamide gel was stained with silver nitrate method (Figure 3).
Column 1: Ladder 100 bp, column 2: negative control, column 3: PAI positive control and internal control (16SrRNA), columns 4 and 5: samples have PAI gene and 16SrRNA, column 6: aer positive control and internal control, columns 7 and 8 of the samples have aer and 16SrRNA, column 9 positive control of traT 16SrRNA, columns 10 and 11 samples have traT and 16SrRNA.
5. Discussion
Escherichia coli resistance to antimicrobial agents is reported all over the world, and the speed of increased resistance has caused concerns about resistance to antibiotics (25). According to the results of this study, the best-recommended antibiotics in cases requiring urgent treatment are amikacin and imipenem, and in subsequent lines, the use of cefipime and nitrofurantoin is suggested. Owing to high resistance to ceftriaxone (45.3%) and ceftazidime (56%), and because resistance genes to broad-spectrum cephalosporins are mainly transmitted through plasmids among bacteria, they can spread among other bacteria (26). Imipenem resistance was 5.3%. Imipenem is a broad-spectrum antibiotic and is stable opposite to most β-lactamases. Imipenem resistance, which is intentionally caused by hydrolyzing enzymes such as carbapenemases, is increasing in Gram-negative bacteria, especially Enterobacteriaceae (27). Therefore, it shows a serious risk that requires more care and further investigation for the presence of carbapenemase enzymes.
In a study on 52 patients with UTI caused by E. coli, Heidari-Soureshjani et al., found 85.71% resistance to ampicillin, 78.78% to nalidixic acid, and 46.51% to ciprofloxacin. The highest degree of susceptibility was observed in nitrofurantoin, amikacin, and gentamicin; this result is consistent with that of our study (28). In Shokri et al. research conducted in Isfahan, Imipenem resistance in E. coli strains was found to be 4%, which is quite consistent with the results of our study. However, resistance to tazocin was reported to be 10%, which is much higher than 1.3% observed in our report (29). In another study, Rahimi et al. examined 194 bacterial susceptibility patterns of UTI caused by E. coli. The resistance rates to ciprofloxacin, ceftazidime, nitrofurantoin, gentamicin, ampicillin, cotrimoxazole, ceftriaxone and nalidixic acid were 1% and 31%, 4%, 28%, 72%, 70%, 32%, and 73%, respectively.
The results are consistent with those of our study with respect to resistance rates of nitrofurantoin, ampicillin, cotrimoxazole, and ceftriaxone (30). In another study carried out in Tehran between 2011 - 2012, 281 cases of E. coli showed the highest rates of resistance to cotrimoxazole, nalidixic acid, and cefazolin, with the highest susceptibility being observed in imipenem, nitrofurantoin, and piperacillin. Resistance rates to cefazolin and cotrimoxazole and the susceptibility to ampicillin and nitrofurantoin are consistent with our reports (31). In another study performed in India in 2017, 351 urine specimens were reported with 43% MDR and the highest antibiotic susceptibility was found in imipenem, nitrofurantoin, amikacin, tazocin and gentamicin, respectively. The strongest resistance to ampicillin, cefuroxime, ceftriaxone (66.58%), and ciprofloxacin (82.85%) was reported, which confirms the results of our study (32). One of the significant points of this study and its comparison with previous studies is that, in antibiotics such as gentamicin, nitrofurantoin, amikacin, and imipenem, the rate of resistance increases with a slower pace compared to other antibiotics such as ampicillin and cotrimoxazole.
In another phase of the current study (Table 2), we examined MDR, Extensive Drug-Resistant (XDR), and Pandrug-Resistant (PDR). Based on MDR pattern provided by Centers for Disease Control and Prevention (CDC) implementation for different antibiotic groups, 16 (21.3%) isolates were MDR and 31 (41.3%) isolates were XDR. According to the definition of CDC, XDR strains were also MDR. Therefore, a total of 47 strains (62.6%) were MDR. Interestingly, although no PDR was observed, the abundance of XDR in the ratio of MDR can be considered a serious alarm, which does not have a good prospect due to the increase in resistance, and causes a serious risk. In a study conducted by Shokri et al. in 2015 in Isfahan, MDR was found to be 79%, which is consistent with our results (25, 29).
In this study, the frequency of aer and traT genes was high in both studied populations. Our results showed that 94% of the isolates had aer gene and 93.3% of them had traT gene in both populations. Also, their frequency was higher in the population with UTI. The results of our study on the frequency of PAI shows that its frequency is lower than that of the aer and traT genes and it is 44% in both populations, which is significant. Contrary to the two other genes, the amount of PAI gene in the healthy population is slightly higher than that of UTI. The high prevalence of these genes reflects the role of host environment in the acquisition and transfer of virulence genes in the bacteria and consequently, the induction of pathogenicity to non-pathogenic bacteria and its transformation into a pathogenic bacterium. In our study, although the prevalence of the three genes studied in both populations was high, there was no correlation between the statistical frequencies of each gene in the two studied groups (P > 0.05).
A research in a center in Iran (Kashan) by Neamati et al. indicated that the frequency rates of aer, PAI, and traT genes in 370 E. coli urine samples were 61.4%, 30.7%, and 74%, respectively, which are lower than the frequency observed in the urine samples of our study (33). Additionally, in a survey of 104 E. coli isolates of UTI by Sholibor, in 2016 in eastern Iran (Zabol), frequencies of traT (38%) and PAI (57%) were reported, which is different from the current study (34). Also, the study of Navidinia et al. was conducted in Tehran in 2013 on 100 isolates of E. coli from UTI (n = 50) and stool of children (n = 50) who were 2 - 12 years old and had no infection. The prevalence of PAI gene was 89% and 38% in the urine and stool, respectively, and their frequency is different from that of our study. This may be due to the difference in geographical area and the small number of samples for comparison or the target group, i.e. children (35).
In a report by Samei et al. on 150 E. coli strains of UTI and 50 strains of stools, the frequency of PAI gene was 98.7% in UTI and 88% in fecal samples (96% in total). Interestingly, MDR was reported at 92.7%, and all MDR patients had PAI gene (36). In a study by Moreno et al. carried out in Barcelona in 2008, there were 109 isolates of E. coli containing 42 isolates of UTI and 67 isolated from fecal samples. The frequencies of traT gene in UTI and fecal were 57% and 42%, respectively (37). In a study performed in Brazil by Oliveira et al. in 2011 on 204 E. coli isolated from UTI, the abundance of aer, PAI, and traT genes was reported 32, 41, and 76%, respectively (38). Moreover, Hassan et al. (2017) carried out a research in Sudan on 150 isolates of E. coli containing 100 isolates of UTI and 50 fecal isolates, demonstrating that the frequencies of aer gene in the isolates from UTI and fecal isolates were 16% and 92%, respectively (39).
Considering the frequency of aer gene in fecal specimens that provides iron absorption for the metabolism of the bacteria in the intestinal environment, the idea that the intestinal environment is a reservoir for pathogen bacteria is augmented (40). TraT is a bacterial gene that is located on the outer membrane and is a common gene of Salmonella, Shigella, E. coli, and Enterobacter. It prevents the impact of complements on the bacteria. Considering its prevalence in most studies on uropathogenic E. coli, this gene can be an interesting suggestive target for therapeutic interventions, an issue that requires further investigation. The presence of the high frequency of PAIs, especially in fecal samples, is important because these genes are easily transmitted and convert a commensal bacteria into a pathogen. Owing to the fact that only the genome of pathogenic bacteria has been noted, little attention has been paid to PAIs in commensal bacteria. Therefore, it is recommended that the subtypes of these PAIs should be explored to gain a better understanding of the commensal strains as a potential pathogenic source. Some of the strains studied in both populations were of the same pattern for the presence of these virulence genes. One of the reasons for this can be the variation of flora E. coli and uropathogenic or the genetic exchange of DNA between strains, which requires further investigation. On the other hand, it is necessary to pay more attention to these pathogenic genes for the purpose of treatment and therapy due to the increasing trend of these genes in the reports compared to previous years.
5.1. Conclusion
Higher degree of the presence of aer and traT virulence factors in strains isolated from urine can indicate the higher potential of UPEC strains compared with fecal strains. High-frequency PAIs genes in E. coli commensal isolates compared with UPEC isolates is important because these genes are easily transmitted and convert some commensal bacteria into a pathogen. This study indicated that ampicillin, cefazolin, and cotrimoxazole should be reevaluated for the treatment of UTI caused by E. coli. In emergency cases, antibiotics such as amikacin, imipenem, and tazocin were used in this area. The study also showed the high rates of XDR and MDR strains among patients, revealing an urgent need to revise the pattern of antibiotic use.