1. Background
Inflammation of the liver parenchyma is called hepatitis. Hepatitis is a gastroenterological disease. This disease can be caused by many factors like viral infections, metabolic dysfunction, alcohol intake, bacteria, and toxins. Sometimes, it is caused by autoimmune diseases. Major factors are alcohol intake, followed by viral infections. Five major viruses causing hepatitis include hepatitis A to E (1). When inflammation persists, it can cause cirrhosis and Hepatocellular Carcinoma (HCC), which are the marked signals for the transplantation of the liver in advanced industrialized countries (2). Approximately 3% of the population worldwide are suffering from this disease, and the diagnosis of new cases reaches about 1.75 million (0.23%) annually. The rate of chronic Hepatitis C Virus (HCV) infection is higher in African Americans than in Hispanic whites and Caucasians. The occurrence of HCV infection is higher in Egypt, Africa, Asia, and China (1, 3). In Pakistan, the seroprevalence varies between 2.2% and 13.5% in different regions. Pakistan is a developing country with a low literacy rate; most people are poorly literate and have the least information regarding diagnostic and therapeutic procedures. Therefore, HCV has become an economic burden for Pakistan, especially Khyber Pakhtunkhwa (4).
Hepatitis C virus belongs to the Flaviviridae family of viruses. The HCV genome is SS + sense RNA. There is a high degree of heterogeneity in sequences, and this heterogeneity is due to the low fidelity of the RNA-dependent RNA polymerase of HCV (5). The HCV genome positive sense RNA strand is copied out to a median negative-sense RNA strand, which is again copied to positive-strand or repeats of the genome. The genome of a positive-strand RNA works as an original template for encoding a polyprotein, which is made up of structural and non-structural proteins. The structural proteins are nucleocapsids and the proteins of envelope E1 and E2 proteins. The NS2-3 protease, the NS3 serine protease, RNA helicase, and NS5B RNA polymerase are the non-structure proteins (6, 7). There are six main genotypes of HCV. In Pakistan, the frequency distribution of HCV genotypes is as follows: 69.1% genotype 3a, 7.1% genotype 1, 4.2% genotype 2, and 2.2% genotype 4 (5).
In the start of chronic hepatitis C management, treatment with non-pegylated and pegylated interferons (Peg-IFN) for two decades and far ahead ribavirin resulted in a decrease in its effectiveness and was fruitlessly tolerated. Between the years 2001 and 2011, the standard of care treatment was the combination of peg-IFN and ribavirin, and the medication period was determined by the genotype of HCV. Generally, the percentage of sustained virological response fluctuated from 40% to 50% with 24 weeks or 48 weeks of combination therapy in genotype 1. Genotype 1a seems slightly more responsive than genotype 1b (8). For genotypes 2 and 3 with 24 weeks of combination therapy with these drugs, the rates of sustained virological response were 60 to 80%. Although the acceptability of peg-IFN was better than that of the non-pegylated types, numerous patients were peg-IFN-intolerant, and ribavirin regularly induced hemolytic anemia and other adverse consequences. Concerns about ribavirin’s teratogenicity are also challenging, which complicates patient management (9, 10). Ribavirin can result in a decrease in ALT levels, but it has little effects on HCV RNA levels (11). Primary protease inhibitors, direct-acting antivirals, telaprevir, and boceprevir successively make available stepwise progress in the rate of sustained virological response.
The introduction of sofosbuvir into the therapeutic regimen has been a landmark success. The highly conserved active site of the NS5B polymerase is targeted by the phosphorylated form of sofosbuvir in the liver, thus terminating the RNA replication of the virus (12). Sofosbuvir, as a direct-acting antiviral drug that is used orally, was discovered for the cure of chronic HCV infection (13). The nucleotide inhibitors are actively functional against all the genotypes; however, the documented data on genotype 5 and genotype 6 is limited. Sofosbuvir is the first NS5B inhibitor to become commercially accessible in early 2014. In addition, sofosbuvir is used in combination with peg-INF plus ribavirin or alone with ribavirin for the treatment of HCV (14, 15).
IL-1β induces the chronic activation of innate immune-mediated inflammation. Chronic activation is triggered by IL-1β and causes innate immune-mediated inflammation (16, 17). Direct-acting antiviral drug therapy has shown to reduce innate immune activity by decreasing the IL-1β production and reducing NFκβ phosphorylation. This leads to a reduction in inflammation with a consequent reduction in liver fibrosis and injury. The reduced expressions of CXCL10 and CXCL11 (chemokines recruiting innate immune cells) were observed by direct-acting antiviral drug therapy.
The normalized function of the NK cell is associated with direct-acting antiviral treatment (18). The secretions of these chemokines decrease as well as the function of NK cells are normalized and are associated with irregular natural immunity setback that leads to the restoration of homeostasis in the natural immune system (19). Alao et al. (20) revealed the upregulation of interferon-stimulated genes (baseline ISG) in those HCV patients who get cured by direct-acting antiviral treatment, indicating that innate immunity plays a role in HCV clearance during direct-acting antiviral treatment. It is noted that the cleavage of MAVS and TRIF by HCV NS3/4A protease inhibits RIG-1 and TLR3 signaling and these two human proteins play a key role in the innate immune response (21, 22). However, the eradication of HCV is not clear. Whether it is caused by NS3/4A protease inhibitors’ direct antiviral effects or by preventing the hydrolysis of TRIF and MAVS, the virus destruction improves the antiviral innate immune response (23).
In Pakistan, INF and ribavirin combination therapy has been used for long periods. That is why many research studies conducted in Pakistan, and even Khyber Pakhtunkhwa, are INF-based therapies (4, 15, 24, 25). Direct-acting antivirals have been just introduced in recent years. As these drugs have just been introduced in Pakistan, especially Khyber Pakhtunkhwa, physicians have switched treatment options to direct-acting antivirals in recent years. Thus, there is limited research that reflects the efficacy and responses of the affected population. Since this therapy is successful in almost all genotypes, we aimed to highlight the response of sofosbuvir-based therapy in different genotypes in chronic HCV patients, particularly in genotypes that were non-responding to previous INF-based therapies in Peshawar, Khyber Pakhtunkhwa.
2. Objectives
The current study was accomplished to evaluate the efficacy of sofosbuvir and weight-based ribavirin combination therapy in those patients who were chronically infected with various genotypes of HCV, with an emphasis on the efficacy of sofosbuvir and weight-based ribavirin combination therapy and its correlation with different host and viral factors (age, liver status, and genotypes) in clinical samples from Peg-INF non-responders and treatment-naïve suspected patients in tertiary care hospitals in Peshawar, Pakistan.
3. Methods
A prospective study was conducted in tertiary care hospitals of Peshawar, Khyber Pakhtunkhwa. The age of the enrolled patients was in the range of 16 - 70 years, and patients were enrolled from January 2016 to March 2017. The study aimed to evaluate the response rate of sofosbuvir and ribavirin therapy in patients who were chronically infected with different HCV genotypes. Samples were collected from suspected subjects. After primary screening with ICT and ELISA, a PCR test was done for each underlying specimen following the manufacturer’s instructions (Roboscreen, Germany). Confirmed anti-HCV subjects with PCR-positive results were selected for the combination therapy, taking into account the exclusive treatment criteria. They took one 400 mg tablet of sofosbuvir orally once a day in combination with ribavirin, based on the patient’s weight (1000 mg/day for < 75 kg and 1200 mg > 75 kg in a divided dose) for six months.
Sofosbuvir has been approved for use in genotypes 1, 2, 3, and 4. The repeated biochemical and PCR tests were done during the course of sofosbuvir and ribavirin combination treatment at weeks 4, 12, and 24. Upon the completion of treatment and confirmation of the sofosbuvir response, samples of all subjects were subjected to PCR testing and liver ultrasound to separate responders and non-responders. The patients were divided into two groups including treatment-naïve and non-responding. These two groups were further divided based on age and the texture of the liver ultrasound. Subjects infected with HCV with age less than 40 years and above 40 years were included in these particular groups
3.1. Statistical Analysis
The study performed a chi-square test to assess whether frequencies in observed groups differed from hypothesized or expected tests.
4. Results
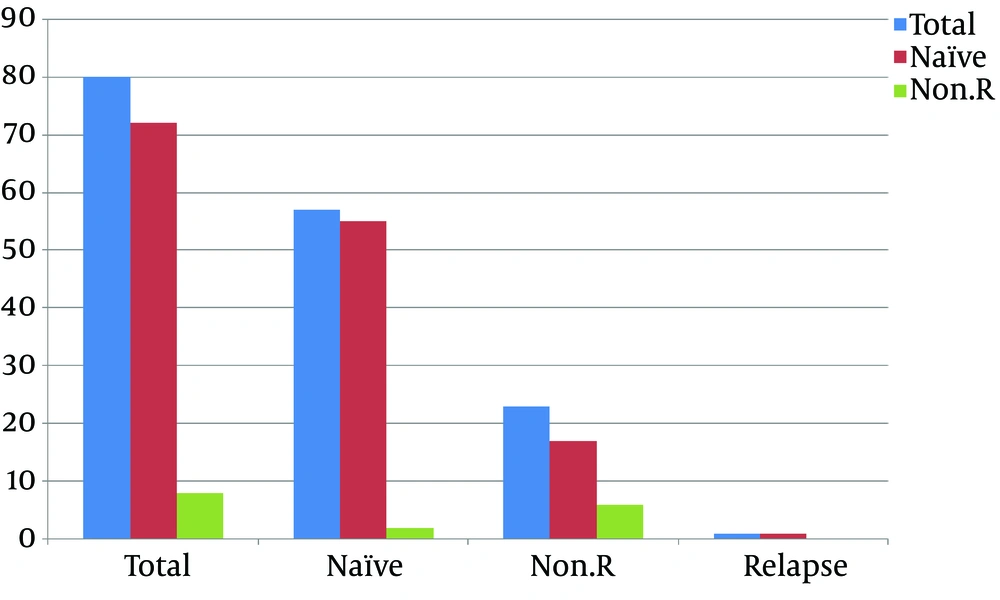
A total of 80 subjects were enrolled in this study. All these subjects were treated with sofosbuvir and ribavirin, and all completed their courses of treatment. The subjects were divided into two groups based on their history. Of the 80 subjects, 57 (71.25%) were treatment-naïve (group 1) and 23 (28.75%) were non-responding (group 2). Further, group 1 was categorized into subgroups according to the results of therapy. In the first subgroup, two (3.5%) subjects did not achieve the end of the therapy response. In the second subgroup, the end of therapy response was achieved in 55 (96.49%) subjects. The next subgroup was based on relapse; one (1.75%) subject relapsed among treatment-naïve subjects (Figure 1). The second group of subjects included non-responders to previous INF therapy; this group included 23 (28.75%) subjects who were non-responders.
Further, this group was categorized into subgroups according to the results of therapy. Thus, six (26.08%) subjects did not achieve the end of therapy response in the first subgroup of the second group. In the second subgroup, the end of therapy response was achieved in 17 (73.91) non-responding subjects. The next subgroup was based on relapse, but none of the subjects relapsed among non-responders.
Regarding gender-wise grouping, 36 (45%) subjects were male of whom 25 (31.25%) and 11 (13.75%) were treatment-naïve and non-responding, respectively. Besides, 44 (55%) subjects were female of whom, 32 (40%) and 12 (15%) were treatment-naïve and non-responding, respectively, as shown in Table 1. The age-wise grouping of enrolled subjects showed that 11 (13.75%) subjects were in the range of 16 - 20 years, 11 (13.75%) of whom were treatment-naïve, and none of them was non-responding. The age of 20 (25%) subjects were in the range of 21 - 40 years, including 10 (12.5%) treatment-naïve subjects and 10 (12.5%) non-responders. The age of 38 (47.5%) subjects were in the range of 41 - 60 years, including 26 (32.5%) treatment-naïve subjects and 12 (15%) non-responders. The age of 11 (13.75%) subjects were in the range of 61 - 70 years, comprising 10 (12.5%) treatment-naïve subjects and one (1.25%) non-responder, as shown in Table 1.
| Factors (Host and Virus) | Naïve (%) | Non-Responding (%) | Total (%) | Chi-Square (Significance)a |
|---|---|---|---|---|
| 1. No. of patients | 57 (71.25) | 23 (28.75) | 80 (100) | |
| 2. Gender | 0.338 | |||
| Male | 25 (31.25) | 11 (13.75) | 36 (45) | |
| Female | 32 (40) | 12 (15) | 44 (55) | |
| 3. Age group (years) | 0.749 | |||
| 16 - 20 | 11 (13.75) | None | 11 (13.75) | |
| 21 - 40 | 10 (12.5) | 10 (12.5) | 20 (25) | |
| 41 - 60 | 26 (32.5) | 12 (15) | 38 (47.5) | |
| 61 - 70 | 10 (12.5) | 1 (1.25) | 11 (13.75) | |
| 4. Ultrasound | 0.179 | |||
| Normal | 30 (37.5) | 12 (15) | 42 (52.5) | |
| CLD | 19 (23.75) | 4 (5) | 23 (28.75) | |
| Hepatoma | None | 2 (2.5) | 2 (2.5) | |
| Cirrhosis | 8 (10) | 2 (2.5) | 10 (12.5) | |
| Fatty Liver | None | 3 (3.75) | 3 (3.75) | |
| 5. Genotypes | 0.043* | |||
| 3 | 41 (51.25) | 10 (12.5) | 51 (63.75) | |
| 2 | 9 (11.25) | 4 (5) | 13 (16.25) | |
| 1 | 1 (1.25) | (1.25) | 2 (2.5) | |
| Mixed | 3 (3.75) | 1 (1.25) | 4 (5) | |
| Un-typed | 3 (3.75) | 7 (8.75) | 10 (12.5) | |
| 6. End of therapy response | 55 (96.49) | 17 (73.91) | 72 (90) | 0.681 |
aSignificance levels: ** at 0.001 and * at 0.05
Liver ultrasound findings showed a normal texture in 42 (52.5%) subjects, including 30 (37.5%) and 12 (15%) treatment-naïve and non-responding subjects, respectively. Chronic liver disease was found in 23 (28.75%) subjects, including 19 (23.75%) and four (5%) treatment-naïve subjects and non-responders, respectively. Liver cirrhosis was found in 10 (12.5%) subjects, including eight (10%) and two (2.5%) treatment-naïve subjects and non-responders, respectively. Fatty liver was found in three (3.75%) subjects, all of whom were non-responders. Hepatoma was found in two (2.5%) subjects, all of whom were non-responders to previous Peg-INF therapy, as shown in Table 1.
Various genotypes’ distribution before treatment included genotype 3 in 51 (63.75%) subjects, including 41 (51.25%) and 10 (12.5%) treatment-naïve and non-responding subjects, respectively. Genotype 2 was found in 13 (16.25%) subjects, including nine (11.25%) and four (5%) treatment-naïve subjects and non-responders, respectively. Genotype 1 was found in two (2.5%) subjects, including one (1.25%) treatment-naïve subject and one (1.25%) non-responding subject. Two mixed genotypes were found in four (5%) subjects, including three (3.75%) and one (1.25%) treatment-naïve and non-responding subjects, respectively. The untyped genotype was found in 10 (12.5%) subjects, including three (3.75%) and seven (8.75%) treatment-naïve and non-responding subjects, respectively, as shown in Table 1.
The results in the age and gender-wise groups showed that the end of therapy response was achieved in 72 (90%) subjects that were treated for 24 weeks with sofosbuvir and ribavirin. The end of the therapy response rate was 32 (88.88%) in males and 40 (90.90%) in females. The end of therapy response rate was higher in females than in males. The rate of end of therapy response in different age groups showed that 17 (85%) and 33 (86.84%) subjects were aged 21 - 40 and 41 - 60 years, respectively. The rate was highest in the age groups of 16 - 20 and 61 - 70 years; it was 33 (86.84%) in the age group of 41 - 60 years and 17 (85%) in the age group of 21 - 40 years, as shown in Table 2. The end of therapy response related to liver ultrasound findings showed that it was achieved in 40 (95.23%) subjects with normal liver texture, 21 (91.30%) patients with chronic liver disease, one (50%) subject with hepatoma, 89 (80%) subjects with liver cirrhosis, and two (66.66%) patients with fatty liver. The end of therapy response rate was higher in those patients who had the normal texture of the liver in ultrasound than in subjects who had abnormal liver textures in their ultrasound, as shown in Table 2.
| Parameters | No. of Subjects | End of Therapy Response Achieved | Age, % | Chi-Square (Significance)a |
|---|---|---|---|---|
| 1. Gender | 0.985 | |||
| Male | 36 | 32 | 88.88 | |
| Female | 44 | 40 | 90.90 | |
| 2. Age groups in years | 0.623 | |||
| 16 - 20 | 11 | 11 | 100 | |
| 21 - 40 | 20 | 17 | 85 | |
| 41 - 60 | 38 | 33 | 86.84 | |
| 61 - 70 | 11 | 11 | 100 | |
| 3. Ultrasound | 0.540 | |||
| Normal texture | 42 | 40 | 95.23 | |
| Chronic liver Disease | 23 | 21 | 91.30 | |
| Hepatoma | 2 | 1 | 50 | |
| Cirrhosis | 10 | 8 | 80 | |
| Fatty liver | 3 | 2 | 66.66 | |
| 4. Genotypes | 0.157 | |||
| 3 | 51 | 49 | 96.07 | |
| 2 | 13 | 10 | 76.92 | |
| 1 | 2 | 2 | 100 | |
| Mixed | 4 | 4 | 100 | |
| Un-typed | 10 | 7 | 70 | |
| 5. End of therapy response | 0.289 | |||
| Total no. of subjects | 80 | 72 | 90 | |
| Treatment-naïve | 57 | 55 | 96.49 | |
| Non-responding | 23 | 17 | 73.91 |
aSignificance levels: ** at 0.001 and * at 0.05
The end of therapy response related to different HCV genotypes showed that it was achieved in 49 (96.07%) patients who were infected with HCV genotype 3, 10 (76.92%) patients who were infected with HCV genotype 2, two (100%) patients with genotype 1, four (100%) patients with the two mixed genotypes, and seven (70%) patients infected with other HCV genotypes, un-typed, achieved end therapy response in subjects, who were treated for 24 weeks with Sofosbuvir and weight base ribavirin.
The rate of end of therapy response was highest (100%) in genotype 1 patients and patients with two mixed genotypes, followed by genotypes 3 and 2, with the rates of 96.07% and 10 (76.92%), respectively. The lowest end of therapy response rate was found in the un-type genotype [7 (70%)], as shown in Table 2.
5. Discussion
The prevalence of HCV is tremendously high in the countryside and surrounding areas of cities. However, one should consider the pint-size of the socioeconomic dimension of the HCV epidemic in Pakistan. Since the mainstream of the Pakistani population lives in these lagging areas of high HCV prevalence, the actual burden of HCV in Pakistan is expected to be much higher (5). This study was designed to assess the outcomes of sofosbuvir therapy combined with ribavirin in HCV patients, particularly concerning its efficacy in those patients who were non-responding to previous INF therapy with different hosts and viral factors in Peshawar, Pakistan. The purpose of our study was to raise awareness and encourage non-responders over the age of 40 to treat HCV with direct-acting antiviral therapy, and practitioners should also consider those patients who, s ages are above 40 years and who are difficult to treat.
Our study achieved a 90% end therapy response, including 96.49% in untreated patients, and 73.91% in non-responders. The rate of HCV prevalence was 55% in women and 44% in men, respectively. Besides, 13.75% of men showed resistance to previous peg-INF therapy. The P value was 0.749, indicating no significant difference between those less than 40 and over 40 years of age among non-responders to previous treatment (Table 1). Most non-responders had an abnormal texture of the liver (Table 1). Another retrospective observational study conducted at the national level showed that dual therapy with sofosbuvir and ribavirin achieved sustained virological response in 81.7% of infected individuals (26). The sustained virological response rate was 94% in patients who received sofosbuvir plus ribavirin (27, 28). The overall sustained virological response was 90% in patients treated with sofosbuvir and ribavirin for 24 weeks (29). However, two recent studies on the genotype 3 Indian population reported sustained virological response-rates of 96% - 98%, regardless of the disease severity and whether the patients were naive or treated (30, 31).
Another study found no significant difference in response to dual treatment with sofosbuvir and ribavirin and it was not affected by host baseline factors, such as age, weight, and extent of liver disease, as well as viral baseline factors such as HCV genotypes (32). In another study, sustained virological response-12 accounted for 81.7% of all patients who were receiving sofosbuvir combination treatment. Response percentages were reduced to 65.5% in the INF non-responder cirrhotic HCV patients who had taken combination therapy (24). Another study from the USA, conducted among treatment-naïve patients who were Egyptians and living in the USA, infected with HCV genotype 4, attained sustained virological response of 100% with dual therapy (33). Similar results were obtained in a phase II study conducted in Egypt, which showed that patients who were naïve to treatment attained a 92% rate of sustained virological response (34).
Our research indicated that the outcomes of treatment depend on the severity of liver disease. The rate of end of therapy response was higher in patients having normal liver texture than in individuals with abnormal liver textures in their ultrasound reports. It was 95.23%, 91.30%, 80%, 66.66%, and 50% in normal, chronic liver disease, cirrhosis, fatty liver, and hepatoma states, respectively. The un-typed genotype and genotype 2 had lower end of therapy response rates as 70% and 76.92%, respectively. We looked at various factors related to the host and the HCV virus to assess their possible impacts on the outcome of treatment. The patient’s age is a key factor in calculating sustained virological response in HCV subjects who had been treated with Peg-INF in combination with ribavirin.
We compared the end of therapy response in patients aged over 40 years and those aged less than 40 years, but it did not discover any remarkable difference between these two groups. The end of therapy responses found in non-responders and untreated patients were 73.91% and 96.49%, respectively (Table 2). Another study revealed that the end of therapy response rate was 95%, and in subjects infected with other than 3a genotypes, the end of therapy response rate was 100%. The rate of response in patients who did not develop cirrhosis was 3.424 folds compared to cirrhotic individuals (32).
International recommendations for 24-week combination treatment with sofosbuvir and ribavirin are suboptimal for cirrhotic patients infected with genotype 3, but not for patients infected with HCV genotype 1 (35). A study conducted at a national level showed a low response rate in cirrhotic patients than in non-cirrhotic individuals (77.2% and 92.3%, respectively). In cirrhotic patients who were non-responders to INF therapy, sustained virological response dropped to 77%. The sustained virological response was 81.7% on average in subjects treated with sofosbuvir and ribavirin (26). Another study revealed that patients infected with HCV genotype 3 in Scandinavian countries were treated with sofosbuvir combined with ribavirin, and even patients with compensated cirrhosis and patients without cirrhosis.
Different HCV drug treatment processes have different end of therapy responses. The achieved end of therapy response was 94% in HCV genotype 2 (36). Another study provided patients infected with HCV genotype 3 with combination therapy with sofosbuvir and ribavirin and achieved an end of therapy response of 96.5% (37). Another study found that among HCV genotype 3 subjects, 91.8% achieved the end of therapy response upon treatment with sofosbuvir and ribavirin (38).
A clinical study by VALENCE showed that in HCV subjects treated with sofosbuvir and ribavirin for 24 weeks, the genotype 3 sustained virological response was 85% (39). A similar study was conducted that 98.92% of patients with genotype 3; 95.65% of patients with HCV genotype 1 and 100% of patients with other HCV genotypes attained the end of therapy response (38). A study conducted in Egypt, HCV genotype 4-infected people attained sustained virological response of 90% (40). In Japan, the sustained virological response-12 rate was 90.4% in patients infected with HCV genotype 2 (41). It is anticipated that by 2030, the overall number of people with HCV viremia will decrease by 90% (42, 43).
The highest HCV problem in the world is tolerated by the country. The existing rate of HCV therapy is 1%, and leadership funds for treating HCV-infected patients are negligible, accounting for 10 million people suffering from HCV in Pakistan. The study was conducted in a city with limited budgetary resources and a small number of subjects, which are the main constraints of the study. In developing countries, awareness of HCV is needed. In underdeveloped countries, the standards for these drugs should remain the same as in developed countries and should be strictly monitored from time to time. In underdeveloped countries, awareness about HCV is necessary. There is a serious demand for diagnosing every person surviving who is infected with HCV and registration in a medication program. In several countries, the core barrier to treatment is the cost of drugs. The majority of therapies are much more expensive.
Financing for hepatitis control and treatment programs is urgently needed because most of the people are poor and illiterate; that is why they have the least awareness about HCV. Proper direction and continuation of therapy are most important in underdeveloped areas, especially Khyber Pakhtunkhwa, Pakistan.
5.1. Conclusions
In this study, females showed a better response to therapy than males. The end of therapy response was not affected by age groups. The abnormal liver texture hepatoma and fatty liver patients had the lowest response to this therapy. The rate of end of therapy response was lower in non-responders to previous therapies than in treatment-naïve HCV patients. HCV genotype 1 had the greatest response to combination therapy with sofosbuvir and ribavirin. The lowest response rate was related to un-type genotypes and peg-INF non-responders’ genotypes. However, the number of patients in this study was small, so it is necessary to investigate the sustained virological response to direct-acting antivirals in various HCV genotypes, particularly in non-responders and un-type genotypes.