1. Background
Urinary tract infection (UTI) is a common disorder which depending on the infection area, various antibiotics can be used (1). According to the 1997 National Hospital Ambulatory Medical Care Survey, UTI accounted for nearly 7 million offices and 1 million emergency department visits, resulting in 100,000 hospitalizations (2). If not treated, this infection causes severe complications such as abnormality in the urinary tract, uremia, hypertension, and, rarely, death (3, 4). Urinary tract infection may also cause septic shock, which is the third-leading infection-caused death (5). Urinary tract infection includes a wide spectrum of clinical entities that are different based on the clinical presentation, degree of tissue invasion, epidemiologic setting, and antibiotic therapy (1, 6). Underlying host factors such as age and gender affect the prevalence of UTI, so it occurs more often in females and males at a ratio higher than 2 (3, 4). Nearly 50 - 80% of females experience a UTI during their lifetime, and 10 to 30% will have frequent infections (1). Complicated UTI, as the leading cause of hospitalization, is associated with high morbidity and increased health expenditures, which imposes a heavy burden on health systems (7-9).
In most cases, intestinal Gram-negative microorganisms are the main pathogen of the UTI (10). The evidence indicates an increasing trend in the resistance of Escherichia coli and other Gram-negative organisms to antibiotics, which are commonly used to treat UTI (7). In patients with complicated infections, antibiotic-resistant Gram-negative bacilli can more commonly be found, compared to those with non-complicated infections (8, 9). The introduction of extended-spectrum beta-lactamases (ESBLs) has intensified antibiotic-resistance by producing Gram-negative bacilli and carbapenems resistance (10, 11). When deciding about antibiotics prescription, the following issues should be in mind: regional resistance pattern, patient-specific factors (including the anatomical location of the infection, the severity of the disease, pharmacokinetics, pharmacodynamics), and costs, especially those related to the medicine (7).
Fosfomycin, first discovered in 1969, was initially used as an intravenous antibiotic to treat various systemic infections. Recently, its oral form is recommended for treating non-complicated cystitis. The absorption rate of Fosfomycin is 58% when fasting, and the rest exerts into the urine. It blocks the synthesis of the cell wall by inhibiting the production of peptidoglycans. Fosfomycin has a broad-spectrum anti-bactericidal activity against Staphylococcus, Haemophilus, and many other Gram-negative bacteria, encompassing 95.5% of the ESBL producing E. coli. Besides, it’s the sole effective antibiotic for 57.5% of ESBL producing Klebsiella pneumoniae and carbapenem producing Gram-negative bacilli. Pseudomonas aeruginosa has a variable sensitivity to fosfomycin, and its minimum inhibitory concentration (MIC) ranges from 4 to 512 µg/mL. However, Acinetobacter baumannii is usually resistant to Fosfomycin. It has excellent in vitro activity against Enterococcus faecalis (97.7%) and E. faecium (100%). Nevertheless, since fosfomycin is mainly used in community-associated infections, the resistance to this effective antibiotic may be incremented (12).
Glycylcyclines are a new class of antibiotics. Tigecycline is the first Glycylcycline, which was approved by the Food and Drug Administration (FDA) in June 2005 to treat complicated skin and intra-abdominal infections. Then, in March 2009, it was approved for treating community-acquired pneumonia. Tigecycline is bacteriostatic in nature and in vitro, it’s effective against a wide spectrum of aerobic and anaerobic bacteria. Also, it’s effective against Enterobactericeae, except for Proteus, Providencia, and Morganella, and is one of the most effective antibiotics against A. baumannii, except for P. aeruginosa. It inhibits the synthesis of protein by binding to the bacterial ribosomal subunit S30. This reaction is reversible (12).
2. Objectives
The rapid growth of Gram-negative bacteria burden, especially Enterobactericeae and A. baumannii, isolated from the urinary tract is conceivably graver than other pathogens, including Gram-positive organisms, since these are common ESBL producers, multidrug-resistant (MDR), or even extensive drug-resistant (XDR). Since such events may have paralyzing consequences, clinicians should prescribe other antibiotics that are effective, have low adverse effects, and are affordable. In this regard, in vitro results are of utmost importance for designing effective treatments. Thus, in the current study, we obtained Gram-negative bacilli recovered from UTI to investigate their susceptibility towards fosfomycin and tigecycline.
3. Methods
3.1. Inoculation and Incubation of Urine Cultures
We investigated all eligible urine specimens that were sent to the Department of Microbiology of the Sina Hospital, which is affiliated to the Tabriz University of Medical Sciences, from April-September 2017. During this period, 2,572 urine specimens were received. Preventing microbiota that causes vaginal, perineal, and anterior urethral infections was the most important reason for collecting urine specimens (13). Thus, in the present study, urine specimens were investigated, which were collected using the urethral catheterization and/or clean-catch techniques (14). Moreover, information on underlying diseases and demographic data were also collected using the medical records of patients.
Past medical history (PMH) was used to determine the existence of underlying urological diseases, and to confirm the findings, the ultrasonography technique was applied. Chronic underlying diseases also were determined based on the PMH. Briefly, each urine specimen was inoculated using a calibrated loop designed to deliver 0.01 mL urine onto eosin methylene blue (EMB) and blood agar (BA) plates semi-quantitatively (14) followed by incubation at 37°C for 24 h. After incubation, the bacterial growth was enumerated. The significant viable count for the diagnosis of bacteriuria was 105 CFU/mL, while specimens with lower counts were also investigated, as described elsewhere (13), depending on the clinical criteria and urine analysis report.
3.2. Bacterial Isolates
The bacterial isolates were identified by growth on EMB agar and blood agar, Gram-staining reaction, and performing biochemical tests, depending on whether the isolate was Gram-positive or Gram-negative. Initially, only Gram-negative bacteria were investigated; thus, to consider differentiation of biochemical reactions (including oxidase test), growth reaction initially on triple sugar Ion agar, SIM (Sulfide, Indole, Motility) medium, MR-VP broth, and citrate utilization tests were used (13). Sugar utilization tests, phenylalanine deaminase, urease, DNase, ornithine, arginine, and lysine utilization tests are other biochemical tests that were incorporated to identify Gram-negative bacteria (15).
3.3. Antibiotic Susceptibility Testing
The antibiotic susceptibility test was accomplished by the disc diffusion method, as described by Kirby-Bauer (16). The susceptibility of the following antibiotics was tested: ciprofloxacin (5 µg), amikacin (30 µg), gentamicin (10 µg), ceftazidime (30 µg), cefotaxime (30 µg), piperacillin-tazobactam (100/10 µg), nitrofurantoin (300 µg), imipenem (5 µg), meropenem (5 µg), co-trimoxazole (25 µg), and levofloxacin (5 µg). All antibiotics were purchased from Mast Diagnostics, UK. To confirm ESBL production, a double-disk test method utilizing cefotaxime and ceftazidime with and without clavulanic acid disks (Mast Diagnostics, UK) was used. Briefly, bacterial suspension was prepared from a 24-hour fresh culture in sterile normal saline, and the turbidity was matched equivalent to 0.5 McFarland. Using the sterile swab, the suspension was inoculated onto Muller-Hinton agar; then, antibiotic disks were placed. The plate was incubated at 37°C for 18 - 24 hours. The inhibitory zone diameter around the disks was recorded. Isolates were classified as susceptible, intermediate, and resistant according to the guidelines published by the Clinical Laboratory Standards Institute (CLSI) (16). Escherichia coli ATCC 25922 and K. pneumoniae ATCC 700603 were used as quality control strains in each set of susceptibility tests.
Based on the standardized international terminology created by the European Centre for Disease Control and Centers for Disease Control and Prevention, the extensively drug-resistant is defined as “non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e., bacterial isolates remain susceptible to only one or two antimicrobial categories)”. While multidrug-resistant is defined as “acquired non-susceptibility to at least one agent in three or more antimicrobial categories” (17).
3.4. Antibiotic Susceptibility of Fosfomycin and Tigecycline
Multi-drug resistant isolates were checked for their susceptibility to fosfomycin and tigecycline. Briefly, bacterial suspension matched equivalent to 0.5 McFarland (equivalent to 1 × 108 CFU/mL) was prepared in sterile saline. Using the sterile swab, the suspension was inoculated onto Muller-Hinton agar, and fosfomycin and tigecycline antibiotic disks (Liofilchem, Italy) were placed on the agar. The plates were incubated at 37°C for 18 - 24 hours. The inhibitory zone diameter around the disks was recorded. CLSI and FDA inhibitory zone diameter breakpoints for Enterobactericeae were used for fosfomycin and tigecycline, respectively, as the breakpoints for tigecycline were not available in CLSI (18, 19):
Fosfomycin: Resistant: ≥ 12 mm, intermediate: 13 - 15 mm and susceptible: ≥ 16 mm.
Tigecycline: Resistant: ≥ 14 mm, intermediate: 15 - 18 mm and susceptible: ≥ 19 mm.
In addition, the susceptibility of antibiotic-resistant isolates towards fosfomycin and tigecycline was confirmed by E-test, through performing inoculation of bacterial cultures as described above for the Kirby-Bauer method and then placing E-test strips (Liofilchem, Italy), instead of antibiotic disks, onto the Mueller Hinton agar plates. CLSI and FDA MIC breakpoints for Enterobactericeae were used to assess the susceptibility of fosfomycin and tigecycline, respectively (18, 19):
Fosfomycin: Resistant: ≥ 256 mg/L, intermediate: 128 mg/L and susceptible ≤ 64 mg/L.
Tigecycline: Resistant: ≥ 8 mg/L, intermediate: 4 mg/L and susceptible ≤ 2 mg /L.
3.5. Statistical Analysis
Data were analyzed using SPSS version 19. Demographic data were analyzed using the descriptive statistics and by providing tables of frequency and mean ± SD. The chi-square statistical method was used to assess the association between quantitative and qualitative variables. The t-test was used to assess the association between quantitative variables.
4. Results
4.1. Positive urine Cultures
Of 2,572 specimens processed, 582 were positive for urine cultures (that is, showed antibiotic resistance). Which, 216 (37.11%) were for males, and 366 (62.89%) were for females. The mean age of males was 61.36 ± 1.23 years, that the youngest and oldest patients were 17 and 94 years, respectively. The mean age of females was 51.20 ± 1.07 years, that the youngest and oldest patients were 12 months and 92 years, respectively. Escherichia coli was the leading pathogen found in urine specimens (67.18%; n = 391). Of these, 71.10% (n = 278) and 28.90% (n = 113) were isolated from females and males, respectively. Klebsiella pneumoniae was the most prevalent pathogen, followed by E. coli, with 9.96% (n = 58) frequency. Of these 9.60% and10.60% were isolated from females and males, respectively. The frequency of other Gram-negative bacteria was as follows: P. aeruginosa (n = 23), A. baumannii (n = 14), Enterobacter spp. (n = 11), and Proteus spp. (n = 1). These pathogens were more frequent in males. In 14.43% (n = 84), Gram-positive cocci was the source of infection, encompassing Enterococcus spp. (n = 68), Staphylococcus aureus (n = 8), S. epidermidis (n = 6), and S. saprophyticus (n = 2) (isolated only from two female patients). Enterococcus spp. was more frequent in females.
4.2. Antibiotic-Resistant Gram-Negative Bacilli
150 antibiotic-resistant Gram-negative bacilli were randomly selected for analyzing antibiotic susceptibility towards fosfomycin and tigecycline. Which, 52% (n = 78) and 48% (n = 72) of isolates were collected from inpatients and outpatients, respectively. The frequency of antibiotic resistance among Gram-negative bacilli separated by admission type and gender is provided in Table 1. Of 150 isolates, 130 E. coli and 11 K. pneumoniae were positive on ESBL testing. Carbapenem resistance was found in 11 isolates, including 5 A. baumannii (1 strain resistant to meropenem, 3 to imipenem and 1 to both antibiotics), 5 K. pneumoniae (all resistant to imipenem), and 1 P. aeruginosa (resistant to imipenem). Two carbapenem-resistant strains were isolated from outpatients and nine from inpatients. Multidrug-resistant and XDR resistance were present in 111 (103 E. coli, 5 K. pneumoniae, 2 P. aeroginosa, and 1 Enterobacter spp.) and 11 isolates (5 A. baumannii, 4 K. pneumoniae, 1 P. aeruginosa, and 1 E. coli), respectively.
| Microorganisms | Total | Type of Admission and Gender | |||||
|---|---|---|---|---|---|---|---|
| Outpatient | Inpatient | ||||||
| Total | Female | Male | Total | Female | Male | ||
| Escherichia coli | 130 (86.67) | 66 | 54 | 12 | 64 | 41 | 23 |
| Klebsiella pneumoniae | 11 (7.33) | 5 | 1 | 4 | 6 | 2 | 4 |
| Pseudomonas aeruginosa | 3 (2) | 1 | 0 | 1 | 2 | 0 | 2 |
| Acinetobacter baumannii | 5 (3.33) | 0 | 0 | 0 | 5 | 1 | 4 |
| Klebsiella (Enterobacter) aerogenes | 1 (0.67) | 0 | 0 | 0 | 1 | 1 | 0 |
| Total | 150 (100) | 72 | 55 | 17 | 78 | 45 | 33 |
aValues are expressed as No. (%).
The frequency of urological and chronic underlying diseases, as well as favorable conditions for UTI in hospitalized patients, is shown in Tables 2 and 3, respectively. The frequency of urinary manipulation in hospitalized patients is described in Table 4. Of 78 isolates of inpatients, 43 (55.12%) had a history of antibiotic consumption over the past three months. The most commonly used antibiotic was ciprofloxacin (23 patients), followed by meropenem (7 patients), ceftriaxone (5 patients), cefixime (4 patients), levofloxacin (3 patients), and cefazolin (3 patients). Besides, cefepime and piperacillin-tazobactam were found in 2 patients, and ofloxacin, gentamicin, azithromycin, cephalexin, and imipenem were found in one patient.
| Underlying Urological Diseases | Total | Gender | |
|---|---|---|---|
| Female | Male | ||
| Renal stone | 19 (24.35) | 11 (24.44) | 8 (24.24) |
| Bladder cancer | 4 (5.12) | 2 (4.44) | 2 (6.06) |
| Bladder stone | 1 (1.28) | 0 | 1 (3.03) |
| Neurogenic bladder | 1 (1.28) | 1 (2.22) | 0 |
| Bladder diverticulum | 1 (1.28) | 1 (2.22) | 0 |
| Polycystic kidney | 2 (2.56) | 2 (4.44) | 0 |
| Benign prostatic hyperplasia | 8 (10.25) | - | 8 (24.24) |
aValues are expressed as No. (%).
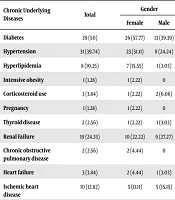
| Chronic Underlying Diseases | Total | Gender | |
|---|---|---|---|
| Female | Male | ||
| Diabetes | 39 (50) | 26 (57.77) | 13 (39.39) |
| Hypertension | 31 (39.74) | 23 (51.11) | 8 (24.24) |
| Hyperlipidemia | 8 (10.25) | 7 (15.55) | 1 (3.03) |
| Intensive obesity | 1 (1.28) | 1 (2.22) | 0 |
| Corticosteroid use | 3 (3.84) | 1 (2.22) | 2 (6.06) |
| Pregnancy | 1 (1.28) | 1 (2.22) | 0 |
| Thyroid disease | 2 (2.56) | 1 (2.22) | 1 (3.03) |
| Renal Failure | 19 (24.35) | 10 (22.22) | 9 (27.27) |
| Chronic obstructive pulmonary disease | 2 (2.56) | 2 (4.44) | 0 |
| Heart failure | 3 (3.84) | 2 (4.44) | 1 (3.03) |
| Ischemic heart disease | 10 (12.82) | 5 (11.11) | 5 (15.15) |
| Valvular heart disease | 1 (1.28) | 1 (2.22) | 0 |
| Coronary artery bypass graft | 1 (1.28) | 0 | 1 (3.03) |
| Cerebrovascular accident | 7 (8.97) | 6 (13.33) | 1 (3.03) |
| Parkinson’s disease | 1 (1.28) | 0 | 1 (3.03) |
| Alzheimer | 3 (3.84) | 1 (2.22) | 2 (6.06) |
| Epilepsy | 5 (6.41) | 1 (2.22) | 4 (12.12) |
| Mental retardation | 1 (1.28) | 0 | 1 (3.03) |
| Ventriculo-peritoneal shunt | 1 (1.28) | 0 | 1 (3.03) |
| Cerebral palsy | 1 (1.3) | 0 | 1 (3.03) |
| Colon cancer | 2 (2.56) | 1 (2.22) | 1 (3.03) |
| Lung mass | 1 (1.28) | 1 (2.22) | 0 |
| Skin disease | 4 (5.12) | 2 (4.44) | 2 (6.06) |
| Burn | 1 (1.28) | 0 | 1 (3.03) |
| Myelodysplastic syndrome | 1 (1.3) | 0 | 1 (3.03) |
| Rheumatoid arthritis | 1 (1.28) | 0 | 1 (3.03) |
| Multiple myeloma | 1 (1.28) | 0 | 1 (3.03) |
| Scleroderma | 1 (1.28) | 1 (2.22) | 0 |
aValues are expressed as No. (%).
| Urinary Manipulation | Total | Gender | |
|---|---|---|---|
| Female | Male | ||
| Urethral catheter | 40 (51.28) | 22 (48.88) | 18 (54.54) |
| Trans urethral lithotripsy | 5) 6.41) | 4 (8.88) | 1 (3.03) |
| Double j stent | 7 (8.97) | 5 (11.11) | 2 (6.06) |
| Prostatectomy | 4 (5.12) | 0 | 4 (12.12) |
| Trans urethral resection of tumor | 4 (5.12) | 2 (4.44) | 2 (6.06) |
| Nephrectomy | 1 (1.28) | 1 (2.22) | 0 |
| Kidney stone surgery | 1 (1.28) | 0 | 1 (3.03) |
| Ureter implant | 1 (1.28) | 1 (2.22) | 0 |
| Supra pubic catheter | 1 (1.28) | 1 (2.22) | 0 |
aValues are expressed as No. (%).
4.3. Antibiotic Susceptibility Towards Fosfomycin and Tigecycline
The results of antibiotic susceptibility of antibiotic-resistant Gram-negative bacilli towards fosfomycin and tigecycline revealed that 92% (n = 138) of isolates were susceptible, and 1.33% (n = 2) were resistant to both. The difference was statistically significant (P = 0.001). Exclusively, 93.33% (n = 140) of isolates were susceptible, and only 5.33% (n = 8) were resistant to fosfomycin, as confirmed by both methods. Also, 96% (n = 144) of isolates were susceptible, while 1.33% (n = 2) were resistant to tigecycline, as confirmed by both methods. Fifty-three patients (67.94%) had a history of urinary system manipulation. Of which, 86.79% (n = 46) and 90.56% (n = 48) of Gram-negative isolates from these patients were susceptible to fosfomycin and tigecycline, respectively. Of 36 isolates obtained from patients with underlying urological diseases, 86.11% (n = 31) were susceptible to fosfomycin, and 94.44% (n = 34) were susceptible to tigecycline.
Of 67 isolates collected from patients with underlying conditions other than urological diseases or predisposing factor to UTI, 91.04% (n = 61) were susceptible to fosfomycin and tigecycline. While, of the 43 isolates obtained from patients with a history of antibiotics consumption during the past three months, 81.39% (n = 35) and 86.04% (n = 37) isolates were susceptible to fosfomycin and tigecycline, respectively. All patients whose isolates were resistant to fosfomycin and/or tigecycline had a history of antibiotic consumption during the past three months. Of ten fosfomycin or tigecycline resistant strains obtained from inpatients, seven patients (70%) had a history of ciprofloxacin consumption. Meropenem (n = 4), ceftriaxone (n = 2), imipenem, cefazolin, and piperacillin-tazobactam were utilized before UTI development. In the absence of the history of antibiotic consumption, all resistant isolates to the common antibiotics were susceptible to fosfomycin and tigecycline.
4.4. Fosfomycin and Tigecycline Resistant Isolates
Information on 12 isolates that were resistant to fosfomycin and/or tigecycline are summarized in Table 5, including 33.33% (n = 4) each K. pneumoniae and A. baumannii, 16.67% (n = 2) P. aeruginosa, and 8.33% (n = 1) each E. coli and K. aerogenes (earlier E. aerogenes). Nearly 75% (n = 9) of these patients were male (P < 0.05) and 25% (n = 3) were female. Most fosfomycin and/or tigecycline resistant strains (66.67%; n = 8) were isolated from elderly patients (older than 50 years) (P < 0.05). About 33.33% (n = 4) of these strains were isolated from patients ≤ 50 years, and the youngest and oldest patients were 29 to 49 years, respectively. Among fosfomycin and/or tigecycline resistant strains, 33.33% (n = 4) were collected from patients hospitalized at intensive care unit (ICU) (P < 0.05), followed by 25% (n = 3) from internal, 16.67% (n = 2) from urology, and 8.33% (n = 1) from the skin ward. Two cases (16.67%) were outpatients. Fifty percent (n = 6) of patients had a urethral catheter (P < 0.05).
| Bacteria | Sex | Age | Type of Admission | Underling Disease | Urologic Disease | Urologic Manipulation | Prior AB Use | ESBL | Carbapenem Resistant | MDR or XDR | Fosfomycin | Tigecycline | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disk Diffusion | E-Test | Disk Diffusion | E-Test | |||||||||||||||
| inhibitory Zone | Result | MIC | Result | Inhibitory Zone | Result | MIC | Result | |||||||||||
| Pseudomonas aeruginosa | M | 29 | Inpatient | Burn | - | Urethral catheter | + | - | - | MDR | 12 | R | N | R | 10 | R | 8 | R |
| Pseudomonas aeruginosa | M | 50 | Inpatient | DM,IHD, epilepsy | - | Urethral catheter | + | - | + | XDR | N | R | N | R | N | R | N | R |
| Escherichia coli | F | 73 | In-patient | - | Urethral stone | stent dj | + | + | - | MDR | 14 | I | 256≤ | R | 24 | S | 0.125 | S |
| Klebsiella (Enterrobacter) aerogenes | F | 70 | Inpatient | Hypothyroidism | Polycystic kidney | Urethral catheter | + | - | - | MDR | N | R | ≥256 | R | 19 | S | 0.5 | S |
| Acinetobacter baumannii | M | 74 | Inpatient | DM, HTN, CRF | Kidney stone | Urethral catheter | + | - | + | XDR | N | R | N | R | 21 | S | 0.19 | S |
| Acinetobacter baumannii | F | 74 | Inpatient | HTN, CVA | Kidney stone | Urethral catheter | + | - | + | XDR | 12 | R | 256≤ | R | 14 | R | 2 | S |
| Acinetobacter baumannii | M | 71 | Inpatient | Parkinson's, Pemphygus, Corticosteroid use | - | - | + | - | + | XDR | 10 | R | N | R | 16 | I | 2 | S |
| Acinetobacter baumannii | M | 46 | Inpatient | Addiction, Epilepsy | - | Urethral catheter | + | - | + | XDR | 21 | S | 48 | S | 32 | S | N | R |
| Klebsiella pneumoniae | M | 91 | In-patient | DM, HTN, IHD | BPH | Prostatectomy | + | + | - | MDR | 17 | S | 12 | S | 13 | R | 3 | I |
| Klebsiella pneumoniae | M | 43 | Inpatient | - | Kidney stone | TUL, stent dj | + | + | + | XDR | 12 | R | 64 | S | 21 | S | 0.38 | S |
| Klebsiella pneumoniae | M | 49 | Outpatient | No data | No data | No data | No data | + | + | XDR | 1 | R | 256≤ | R | 19 | S | 1 | S |
| Klebsiella pneumoniae | M | 54 | Outpatient | No data | No data | No data | No data | + | + | XDR | N | R | 256≤ | R | 19 | S | 1 | S |
Abbreviations: AB, antibiotic; BPH, benign prostatic hyperplasia; CRF, chronic renal failure; CVA, cerebral vascular accident; dj, double j stent; DM, diabetes mellitus; HTN, hypertension; I, intermediate; IHD, ischemic heart disease; N, no zone; R, resistant; S, susceptible; TUL, trans urethral lithotripsy.
All fosfomycin and/or tigecycline resistant strains were also resistant to ciprofloxacin, levofloxacin, ceftazidime, cefotaxime, and piperacillin-tazobactam. Ninty one point sixty six percent (n = 11) of them were resistant to gentamicin and trimethoprim-sulfamethoxazole. 83.33% (n = 10) were resistant to nitrofurantoin, 75% (n = 9) to amikacin, and 66.67% (n = 8) to imipenem. All strains were susceptible to colistin. Concerning the resistance profile, 66.67% (n = 8) of isolates showed XDR profile (P < 0.05), while 33.33% (n = 4) were MDR. About 66.67% (n = 8) and 41.67% (n = 5) were phenotypically resistant to carbapenems (P < 0.05) and ESBL-producing, respectively. Among fosfomycin and/or tigecycline resistant isolates, only P. aeruginosa isolates were resistant to both antibiotics when tested by disk-diffusion and E-test methods.
5. Discussion
In this study, isolates collected from females were significantly higher than males. The mean age of male patients was ten years higher than females. The lower mean age of females can be attributed to the high prevalence of UTI among females in the reproduction age. As reported by various studies, UTI is more prevalent among females than males (3, 4). The most common chronic disease was diabetes mellitus. Regarding the glucose excretion in diabetic patients, this underlying disease may be a predisposing factor for the onset of an infection. The high comorbidity of UTI with diabetes in females can be attributed to the higher prevalence of UTI among females. A study that investigated the therapeutic consequences of tigecycline for UTI caused by carbapenem-resistant Enterobactericeae, has mentioned to diabetes and renal failure as the most common underlying diseases (20). The most common manipulation was urinary catheter insertion, which is consistent with the results of various studies that reported manipulation of the urinary system, including urinary catheter insertion, as a risk factor for UTI (12). The most commonly used antibiotic was ciprofloxacin, with a frequency of 53.48%. In the study by Krueger et al. (21), 66% of patients had a history of antibiotic consumption, and ciprofloxacin was prescribed for 67.99% patients.
Treating MDR and XDR Gram-negative infections, including A. baumannii, is still an unresolved issue because almost all aforementioned observations resulted from small case series (22-24). Shokri et al. (25) found that the tigecycline and fosfomycin are the most effective antibiotics against K. pneumonia and P. aeruginosa, who had New Dehli Metallo-β-lactamase, respectively, in Iranian patients. Moreover, a study recently conducted in Iran reported the effectiveness of tigecycline against K. pneumonia (26). According to an evidence-based review, carbapenem-resistant Gram-negative pathogens were the most challenging issue and antibiotic regimen, including carbapenem plus tigecycline or colistin, were the most effective therapeutic option in low-level resistance cases (27).
In the current study, the most prominent pathogen was E. coli, with a frequency of 86.67%, which is consistent with an earlier investigation that found a high frequency of E. coli in 80% of females and 20% males (28). A study by Sultan et al. (29) mentioned to E. coli as the most common pathogen, with an incidence of 80.8% and a frequency of 79.7% among outpatients (29). Two studies on UTI found E. coli as the prevalent etiological organism and isolates were resistant to common antibiotics (30, 31). In the current study, which investigated 150 antibiotic-resistant Gram-negative bacilli, the highest antibiotic susceptibility was for colistin (97.80%), followed by amikacin (86%) and imipenem (85.50%). While the highest antibiotic resistance was towards cefotaxime (95%), followed by ceftazidime (94.10%), ciprofloxacin (90.70%), trimethoprim-sulfamethoxazole (87%), and levofloxacin (86.90%). In an earlier study conducted in Tabriz, the highest drug resistance was towards ampicillin, cefazolin, nalidixic acid, trimethoprim-sulfamethoxazole, cefotaxime, and ciprofloxacin (32).
Hernandez et al. (33) reported the susceptibility of ESBL producing isolates towards fosfomycin as 97.4%. Garau (34) investigated 428 ESBL producing Gram-negative bacilli and found that 97.4 and 97.5% of isolates were susceptible to fosfomycin and tigecycline, respectively. A study performed in Turkey has investigated the administration of fosfomycin for treating lower UTI induced by ESBL producing E. coli and reported a success rate of 94.3% and 78.5% microbiological success (35). Auer et al. (10) in a study which tool for four-years (2004 to 2008) showed a high in vitro activity of fosfomycin on ESBL producing E. coli (97% E. coli were sensitive to fosfomycin). Meier et al. (36) conducted a study on the community-acquired ESBL producing E. coli from the urine specimens, and found similar positive outcomes for fosfomycin, while found high resistance to many traditional oral antibiotics.
A study performed in Spain, which its results are similar to the current study, reported that > 90% E. coli and Citrobacter and > 70% of K. pneumoniae, Enterobacter spp., Proteus mirabilis, S. aureus, Coagulase-negative staphylococci, and Enterococcus spp. isolated from the urine were susceptible to fosfomycin (37, 38). Korean and UK studies provided similar results with 87.7%, and 95.1% of E. coli and K. pneumonia, respectively, were susceptible to fosfomycin (39). Geerlings et al. (40) reported two patients with frequent UTI episodes with ESBL producing E. coli that were successfully treated with tigecycline. In a systematic review by Brust et al. (20), 14 patients with UTI caused by MDR Gram-negative bacilli were treated successfully after the administration of tigecycline. In a study conducted in the cities of Tabriz and Urmia on UTIs, 97.3% of MDR and ESBL producing isolates were susceptible to fosfomycin (32); however, these studies did not provide clinical details and parameters related to the fosfomycin resistance are not compared. This is a clinical-microbiological study aimed to evaluate the susceptibility or resistance of Gram-negative bacilli towards fosfomycin and tigecycline which its results are consistent with the above-mentioned studies.
5.1. Conclusions
Appropriate and effective treatment of UTI requires awareness about both epidemiology and antibiotic resistance patterns. In the present study, significant resistance to the conventional antibiotics prescribed for treating UTIs was found. Based on the results, fosfomycin and tigecycline had appropriate antimicrobial activity towards Gram-negative resistant isolates involved in UTIs. Despite the low resistant rates of Gram-negative bacilli to fosfomycin and tigecycline, it seems uncontrolled antibiotic consumption (arbitrarily or non-purposeful) has a significant role in the emergence of resistant strains. Also, a significant association was found between urinary catheter insertion and UTIs.