1. Background
Obesity is a medical condition associated with excessive accumulation of fat, which leads to weight gain with a body mass index (BMI) of 30 kg/m2 or above. As a metabolic disorder caused by excess triglycerides in adipose tissue, obesity can induce inflammatory mediators, such as leptin, resistin, chemical mediator monocytes (MCP-1), and tumor necrosis factor (TNF-α). Cytokines are small soluble molecules involved in intercellular communication. They are produced by a wide spectrum of body cells, including the liver (1). These molecules have several subfamilies, including interferons, growth factor transformers (TGFs), tumor necrosis agents (TNFs), interleukins (INFs), colony stimulants, and chemokines (1). Cytokines are responsible for several basic biological processes, including body growth, obesity, lactation, bleeding, as well as inflammation, and immunity. However, they are involved in various pathologies, for instance, rheumatoid arthritis atherosclerosis, nonalcoholic fatty liver disease (NAFLD), psoriasis, and systemic lupus erythematosus (2, 3). The production of cytokines in the liver is absent or minimal under physiological conditions. However, pathological stimuli (e.g., fat accumulation) stimulate liver cells to produce such inflammatory molecules. Cytokines are believed to be essential in the development of nonalcoholic fatty liver disease by stimulating hepatitis, cell necrosis, and apoptosis, as well as induction of fibrosis. Nonetheless, they have a pivotal role in liver regeneration following injuries (1, 3). The pathophysiological role of cytokines in NAFLD has also been discussed.
Several cytokines play a key role in triggering acute inflammatory interactions, including IL-1, IL-8, IL-11, IL-6, and TNF-α. Of which, IL-1 and TNF-α have the greatest inflammatory induction power. As an inflammatory mediator, several inflammatory cells can secret TNF-α, including macrophages, neutrophils, monocytes, and T cells. At the same time, TNF-α is secreted by many other tissues, such as endothelium, adipose tissue, or nervous tissues. In the liver, it is secreted directly by hepatocytes and Kupffer cells or indirectly by abdominal fat (4).
Transforming growth factor-beta (TGF-β) is a cytokine with anti-inflammatory features (5). In the liver, TGF-β1 secreted by immune cells, stellate cells, and epithelial cells is the most abundant isoform. It plays a considerable role in hepatic fibrosis by activating star cells and producing extracellular matrix proteins (6).
The role of interleukin 6 (IL-6) in liver pathology is highly complex, and its contribution to the development of NAFLD is not clear yet. IL-6 activates several cells, such as immune cells, liver cells, blood stem cells, and osteoclasts. In addition, IL-6 is of a wide range of biological functions, including inducing inflammation and inclusion, regulating the immune response, and supporting bleeding (7).
Today, owing to the successful effects of synthetic drugs, the use of these drugs has increased. Even though the use of anti-fat chemical drugs, such as gemfibrozil, clofibrate, and statins, reduces blood lipids and cardiovascular complications, synthesis problems, high cost, and side effects, have made medicinal plants and traditional medicine more popular among the public and people are more inclined to use this method. There is evidence regarding the certain properties of some medicinal plants, such as controlling oxidation, regulating blood lipids, and reducing inflammation (8, 9).
Aloe vera is a natural product that can prevent obesity-related metabolic disorders. It also can reduce the accumulation of fat by its role in preventing obesity-related metabolic changes and antioxidant effects. In addition, Aloe vera acts as a practical food in activating fat lipolysis and preventing obesity-associated metabolic changes (10).
2. Objectives
In this line, the present study aimed to evaluate the effect of Aloe vera alcoholic extract on inflammatory cytokines of rats fed with a high-fat diet.
3. Methods
3.1. Preparation of Aloe vera Extract
To prepare the alcoholic extract of Aloe vera, fresh leaves of Aloe vera were prepared, and their type and species were determined by botanical experts at the Botany Department of Shiraz University. Subsequently, before removing the gel, the leaves were washed. Afterward, the gel was mixed with 95% ethanol (1:4) in a container. Then, it was placed on a shaker for 4 days, then filtered and concentrated at 45°C using a rotary evaporator. It was finally completely dried at 40°C and powdered (11).
3.2. Animals and Grouping
In the present study, 40 male Wistar rats (200 g - 220 g), obtained from Shiraz University of Medical Sciences, were studied. All animals were fed using compressed food (pellets), prepared from livestock and poultry feed company. Tap water from the Shiraz municipal system was used as the source of animal drinking water. The water was provided in plastic containers as drinking water. All animals were kept in transparent macrolone cages (55 × 30 × 55 cm) with a steel mesh roof, and the floor of the cages was covered with sawdust and wood chips. It is noteworthy that the wood chips in the cages were replaced every two days, and the cages were washed and disinfected with alcohol and cetrimide-C 250ML concentrated solution (savlon). The ambient temperature and relative humidity were 22 ± 2°C and 55 ± 3%, respectively, with a 12-hour light/12-hour dark cycle. The floor and equipment were frequently disinfected with savlon. The rats were randomly divided into five groups, as follow:
1- Control Group: They were stored for 60 days under normal conditions without receiving any additional substances.
2- Control Group Receiving a High-Fat Diet (HFD): In this group, the animals received the same volume of solution given to the experimental groups, solvent extract (physiological serum) + high-fat emulsion by gavage.
3- Experimental Group 1: They were treated with normal food and high-fat emulsion for 60 days and received a dose of 150 mg/kg body weight of Aloe vera extract simultaneously.
4- Experimental Group 2: They were treated with normal food and high-fat emulsion for 60 days and simultaneously received a dose of 300 mg/kg body weight of Aloe vera extract.
5- Experimental Group 3: They were treated with normal food and high-fat emulsion for 60 days and at the same time received a dose of 600 mg/kg body weight of Aloe vera extract (12).
3.3. Preparation and Administration of a High-Fat Diet
To implement the HFD protocol in the present study, the high-fat emulsion protocol used in the previous studies in 2016 and 2006 (13, 14) was adjusted. The duration of administration and treatment in different groups was 60 days. The high-fat emulsion was administered by gavage at a rate of 1 ml each time in the mornings and evenings. Table 1 represents the compounds employed in the high-fat emulsion protocol in the present study (12).
3.4. Blood Sampling and Serum Preparation
Anesthesia was performed by ketamine injection. Following anesthesia, the tip of the syringe was entered the animal’s left ventricle from the chest and left side of the animal’s body to let the blood enter the syringe tank. The blood tubes were placed at room temperature for 30 minutes until the blood was completely clotted. The tubes were then centrifuged at 3500 rpm for 10 minutes to separate the serum (CENTRIC 260R model made by Domel Europe). Afterward, a sampler and a supervisor separated the serum and poured it into separate tubes. Then, all tubes were frozen at -20°C until measuring target factors.
3.5. Laboratory Tests on Blood Serum Samples
EAST BIOPHARM ELISA kit was utilized to measure TNF-α, TGF-β-, IL-6, and INF-γ.
3.6. Statistical Analysis Procedure
Data analysis was administered using SPSS-20 software by ANOVA test. Furthermore, the significance of data differences was measured via Duncan’s multiple range test. Statistical significance was considered when P-value ≤ 0.05.
4. Results
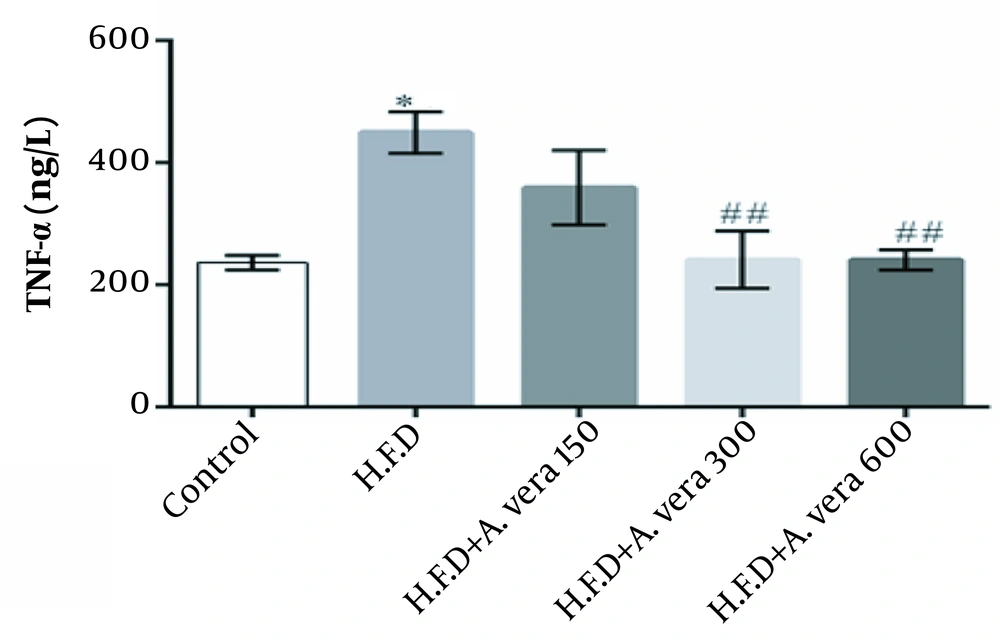
The findings regarding the comparison of the mean concentration (TNF-α) in the studied groups of adult male Wistar rats are depicted in Figure 1.
Comparison of the mean concentration of TNF-α in the studied groups of adult male Wistar rats. *, The high-fat diet group was significantly different in the control group (P ≤ 0.05). There were no significant differences between the high-fat diet + Aloe vera alcoholic extract group (at dose 150) compared to other groups P ≤ 0.05; ##, Indicates a significant difference between study groups that received a high-fat diet + Aloe vera alcoholic extract (at doses 300 and 600 mg/kg) compared to the group that received a high-fat diet (P ≤ 0.01).
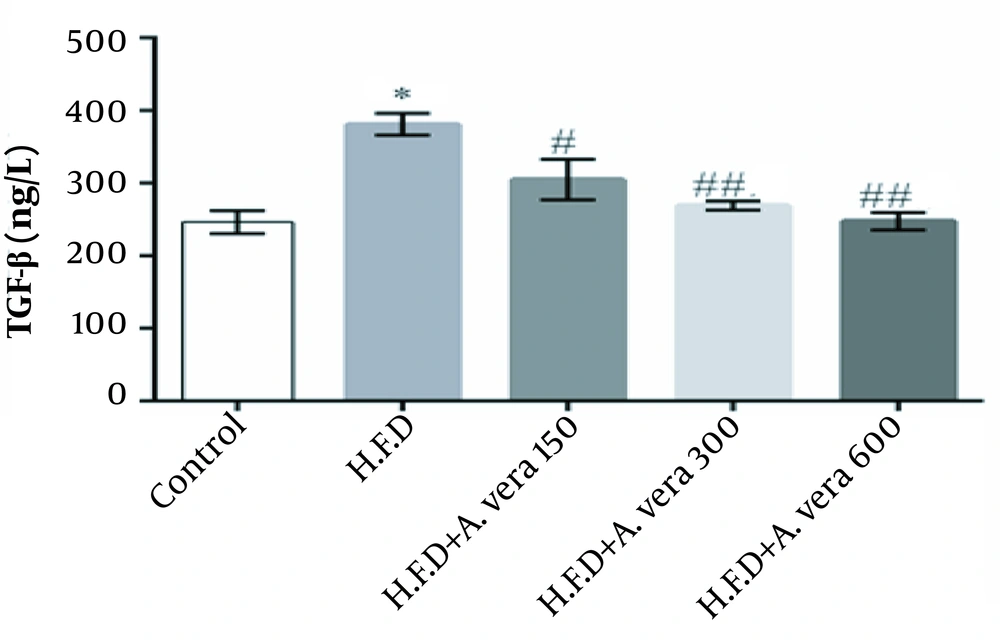
The results of the comparison of the mean concentration of TGF-β in the studied groups of adult male Wistar rats are depicted in Figure 2.
Comparison of the mean concentration of TGF-β in the studied groups of adult male Wistar rats. *, Indicates a significant difference between the HFD and the control groups (P ≤ 0.05); #, Indicates a significant difference between the group receiving 150 mg/kg body weight Aloe vera + HFD compared to the group receiving a high-fat diet (HFD) (P ≤ 0.05); ##, Indicates a significant difference between the groups receiving 300 and 600 mg/kg body weight of Aloe vera + HFD compared to the group receiving a high-fat diet (HFD) (P ≤ 0.01).
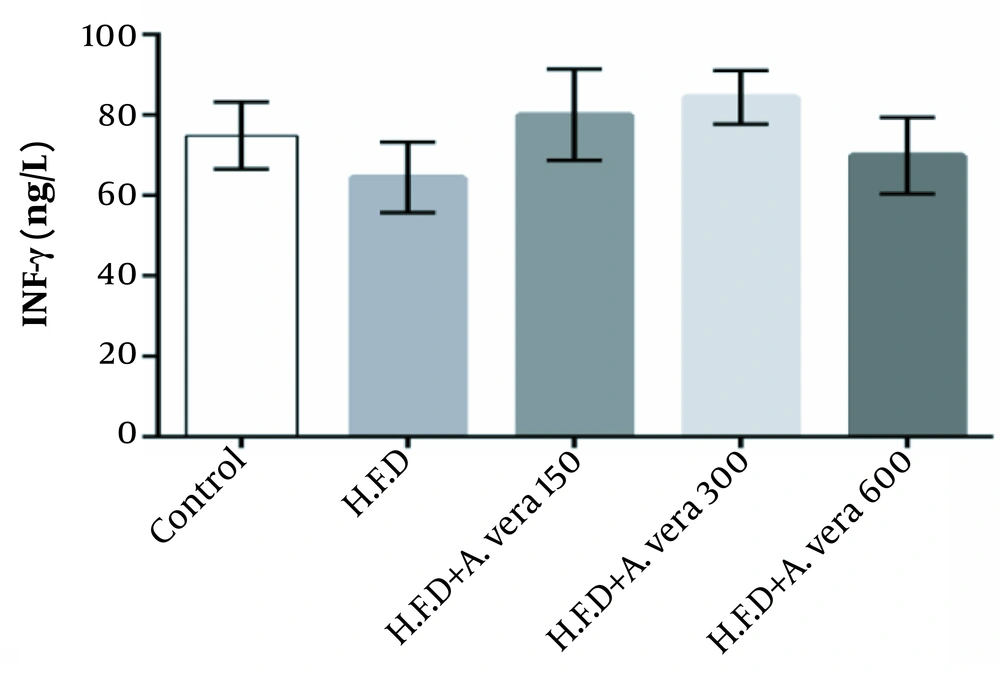
The results of the comparison of the mean concentration of INF-γ in the studied groups of adult male Wistar rats are exhibited in Figure 3.
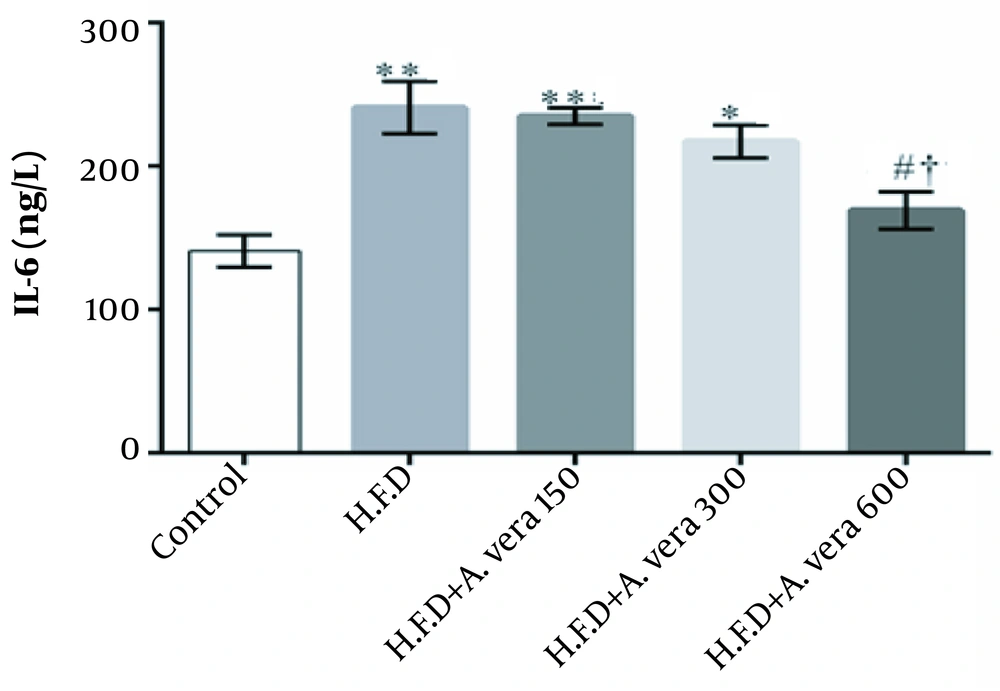
The findings regarding the comparison of the mean concentration of Interleukin-6 (IL-6) in the studied groups of adult male Wistar rats are represented in Figure 4.
Comparison of the mean concentration of IL-6 in the studied groups of adult male Wistar rats. **, There was a significant difference among the high-fat diet group and high-fat diet + Aloe vera alcoholic extract group and the control group at a dose of 150 mg/kg (P ≤ 0.01); *, There was a significant difference between the high-fat diet + Aloe vera alcoholic extract group and the control group at a dose of 300 mg/kg (P ≤ 0.05); #, Indicates a significant difference between the groups receiving a high-fat diet + Aloe vera alcoholic extract at a dose of 600 mg/kg) compared to the group receiving a high-fat diet (P ≤ 0.05); †, Indicates a significant difference between the group receiving a high-fat diet + Aloe vera alcoholic extract at a dose of 600 mg/kg) compared to the Aloe vera alcoholic extract-receiving group at a minimum dose (P ≤ 0.05).
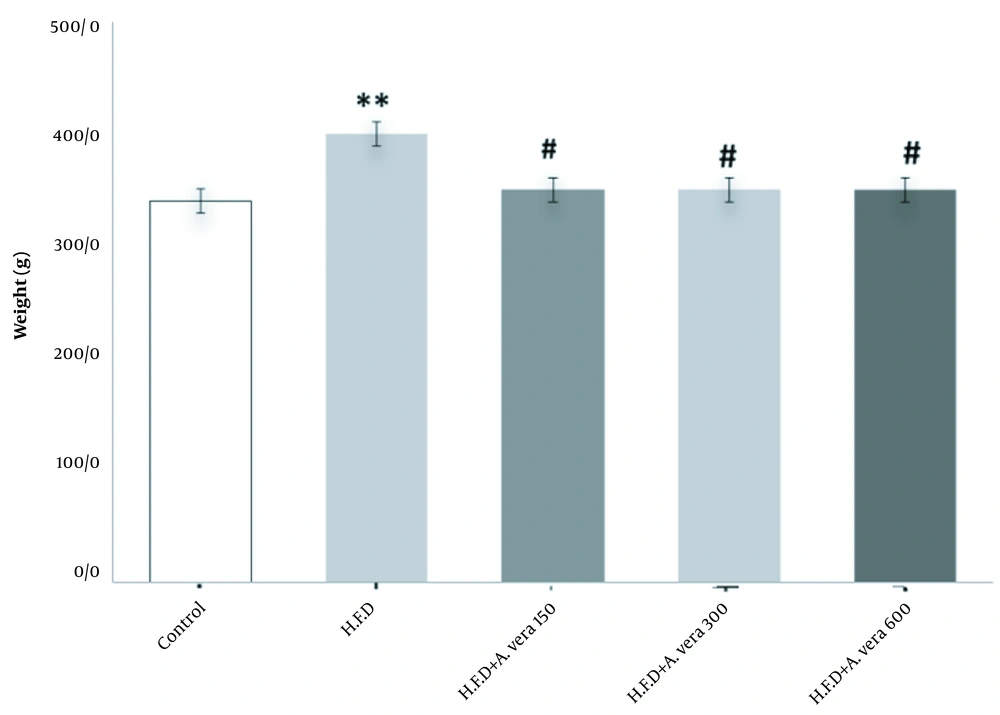
The results related to the comparison of the mean concentration weight(g) in the studied groups of adult male Wistar rats are shown in Figure 5.
Comparison of the mean concentration of weight (g) in the studied groups of adult male Wistar rats. *, Indicates a significant difference between the HFD group and the control group (P ≤ 0.05); #, Indicates a significant difference between the group receiving 150, 300, and 600 mg/kg body weight Aloe vera + HFD compared to the group receiving a high-fat diet (HFD) (P ≤ 0.05)
5. Discussion
This study demonstrated that the high-fat diet could increase the concentration of tumor necrosis factor-alpha (α) TNF, TGF-β, and interleukin 6 (IL-6) in the high-fat diet (HFD) group compared to the control group, and no considerable changes were observed in the mean concentration of INF-γ in the studied groups. Meanwhile, administration of Aloe vera gel extracts reduced the concentration of tumor necrosis factor-alpha (α- TNF), TGF-β, and interleukin 6 (IL-6) in the groups receiving a high-fat diet + Aloe vera alcoholic extract compared to the group receiving a high-fat diet (HFD).
A study conducted in 2019 reported that the accumulation of lipids in the kidneys is known as renal fat and causes renal lipotoxicity. Renal lipotoxicity results in damage to kidneys, including increased pro-inflammatory factors, insulin resistance, degradation of lipid metabolism, or inactivation of the renin-angiotensin-aldosterone system. The proliferative activator receptor for gamma peroxisome isoform (PPARγ) is believed to play an important role in the development of this lipotoxic (15).
According to the above-mentioned study and the findings of the present study, tissue damage and inflammation can be reduced considerably through controlling the overweight and reducing the effects of damage to high-fat foods; these studies mentioned this role for Aloe vera extract. In this regard, Yazdani and Hosseini (12) reported that a high-fat diet could increase body weight and serum levels of triglycerides, cholesterol, and LDL. They also reported no considerable effect on serum levels of HDL. Meanwhile, Aloe vera gel extract improves the lipid profile and weight loss of animals treated with a high-fat diet (12).
Additionally, a study conducted in 2019 reported that oral consumption of Aloe vera extracts has anti-diabetic and anti-obesity effects. This study also reported that rats fed with a high-fat diet treated with Aloe vera extract experienced lost body weight and liver weight as well as suppression of fat (16).
In the same vein, another study found that administration of Aloe vera gel at 100 and 200 mg/kg per day prevents the accumulation of adipose tissue and corrects dyslipidemia. The protective role of Aloe vera gel against obesity-related metabolic changes and its antioxidant properties cause reduced fat accumulation. Aloe vera, as a functional food, has great potential in activating fat lipolysis and preventing metabolic changes associated with obesity (10).
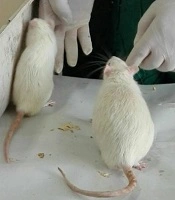
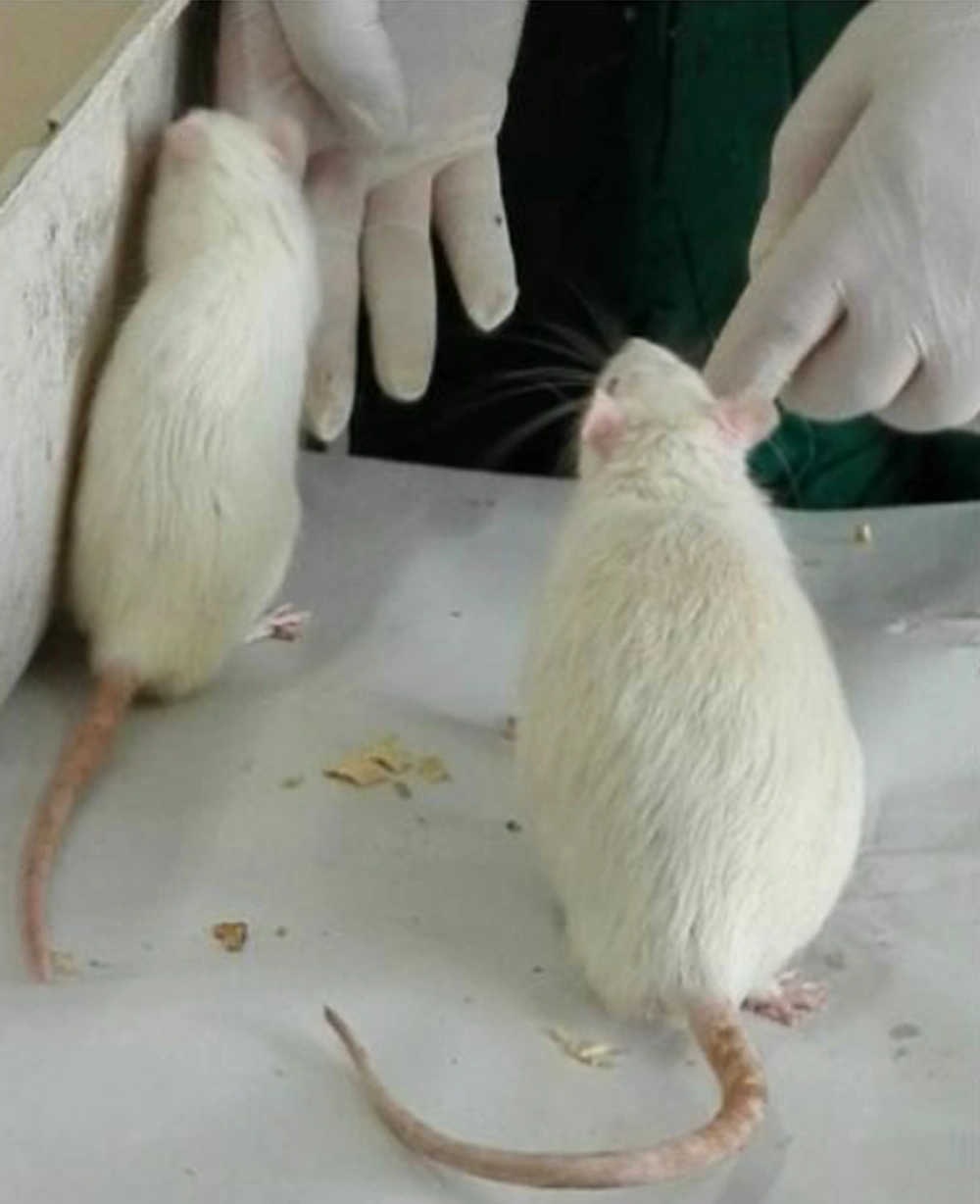
The reduction of body fat accumulation during regular consumption of Aloe vera was clearly observed in our research, as shown in the image below. In this image, the rat on the right was treated with a high-fat diet for 60 days, and the left one received a high-fat diet with Aloe vera at a dose of 600 mg/kg body weight. Weight gain in the rat that did not receive Aloe vera is obvious (Figures 5 and 6).
In obese people with diabetes or those under early treatment for diabetes, Aloe vera complex reduces body weight, body fat mass, and insulin resistance (17). Consistent with the present study, it has been shown that Aloe vera gel with Acemannan compound improves lipid profile in patients with diabetes (12, 18).
Aloe vera gel extract may improve the lipid profile of high-fat diet rats by acting on the expression of genes involved in fatty acid metabolism. Vitamin E (alpha-tocopherol) is an antioxidant that protects cell unsaturated fatty acids against lipid peroxidation and free radicals produced during this reaction and stabilizes cell membranes. In addition, it has antioxidant effects that inhibit the progression of nonalcoholic fatty liver disease (19). It is worth noting that one of the ingredients of Aloe vera gel extract is vitamin E (20). Therefore, in the present study, Aloe vera gel extract, which contains vitamin E, could modify the inflammatory factors in animals treated with a high-fat diet. Furthermore, Aloe vera has anti-inflammatory and antioxidant effects that prevent neutrophil migration and the production of pro-inflammatory signals (21). A study has provided evidence that confirms phytosterols isolated from Aloe vera gel can reduce visceral fat mass and lower blood sugar (22).
Aloe vera leaves are rich in anthraquinone compounds. In addition, its gel, which also uses as food, has several beneficial properties, including anti-inflammatory, antioxidant, anti-virus, antibacterial, and wound healing. Furthermore, it has characteristics that result in lowering fat and blood pressure, and are anti-diabetic, anti-obesity, and heart-protecting (23). Anthraquinone is an active ingredient in Aloe vera. Aloe imodine and barbaloin, which are anthraquinones, also are available in Aloe vera (17). Anthraquinone acts as an antioxidant in mediated reactions during inflammatory responses and is conducive to necrosis treatment (24). Probably due to this anti-inflammatory property, in the present study, animals that received Aloe vera extract had reduced TNF-α and IL-6.
A previous study has evaluated the effects of aloe-iodine on innate immunity and immune cytokines in renal leukocytes. In the present study, animals fed with 5 mg aloe-emodin had significantly increased levels of albumin and globulin after six weeks. Expression of pro-inflammatory cytokines, such as IL-1β, IL-8, TNF-α, and iNOS, was considerably modulated during feeding with 5 mg aloe-emodin in the eighth week (25). Since aloe imodine is an anthraquinone compound found in Aloe vera, it has been suggested as a protector for oxidation and free radical activities (26). Therefore, the reduction in the disorders caused by a high-fat diet, including the reduction in pro-inflammatory factors and the increase in albumin levels, could be attributed to the presence of aloe immodin in Aloe vera extract.
5.1. Conclusions
This study demonstrated that a high-fat diet increased the density of inflammatory factors in the control group. No considerable effects were observed on the mean concentration of INF-γ in the studied groups while Aloe vera gel extracts considerably reduced inflammatory factors TNF-α, TGF-β-, and IL-6. It should be noted that this study was performed on animals and whether this substance has similar effects on humans or not requires further study.