1. Background
Aflatoxins are mycotoxins produced by Aspergillus flavus (1). They are toxic substances and have carcinogenic property for human and animal (2). Aflatoxins are found in improperly stored chief products such as cassava, chili peppers, corn, cotton seed, millet, peanuts, rice, sorghum, sunflower seeds, tree nuts, wheat and a variety of planets and relative products. The most toxic aflatoxin is aflatoxin B1 (AFB1) that is easily produced on feed and food during growth and storage (2-4). Animals are exposed to these mycotoxins by consumption of feeds contaminated with mycotoxin-producing molds during growth, harvest and/or storage. The AFB1 can potentially be converted to aflatoxin M1 (AFM1) by cytochrome P450 enzyme system in liver (5). AFM1 is a hydroxylated metabolic form of AFB1 usually excreted in milk through the mammary gland (6). AFM1 can increase the risk of liver cancer in human (4). It is also resistant to autoclaving, pasteurization and thermal inactivation (7). Carcinogenicity of AFB1 is ten times more than that of AFM1 (8). For this reason, AFB1 was considered as the primary and AFM1 as the secondary groups for carcinogenic compounds by the International Agency for Cancer Research (9). Milk and its products are important nutrients for population, but if contaminated with AFM1 or AFB1 they can endanger public health. Therefore, it is quite vital to determine both the presence and level of these mycotoxins in milk and dairy products.
2. Objectives
The present study aimed to evaluate AFM1 levels in 97 samples of raw milk and 20 samples of pasteurized milk by high-performance liquid chromatography (HPLC).
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
The AFM1 standard was purchased from Supelco (USA); acetonitrile and methanol were HPLC grade from Merck (Darmstadt, Germany). All buffers were prepared from deionized water.
3.2. Samples
Ninety-seven samples of raw milk and 20 samples of pasteurized milk were collected from milk collecting centers and different supermarkets with several brands respectively from January to March 2014.
These samples were transferred to in the Fardis central laboratory of food and drug. All samples were refrigerated and analyzed before expiration date.
3.3. Apparatus
The levels of AFM1 were determined by isocratic revers-phase liquid chromatography using a Shimadzu HPLC system (Japan) equipped with a Shimadzu RF-20A florescence detector, LC-20AD pump, DGU-20A degasser, CTO-20-A system controller and LC solution software. The HPLC analysis was performed using C18 column (4.6 × 250 mm) which was purchased from Hector RSTCH Corporation. Excitation and emission wavelengths were selected at 365 and 435 nm, respectively. Immonoaffinity column was prepared from Libios company (France).
3.4. Determination of AFM1
Milk samples were analyzed for the presence of AFM1 using an immunoaffinity column for cleanup and an HPLC machine with a florescence detector.
3.5. Sample Preparation
The milk samples (40 mL each) were centrifuged at 3500 × g for 10 minutes and upper fat layer was removed. The remained skim milk was passed through a filter paper (Whatman No.4). Then, 20 mL of the skimmed milk was passed through an immunoaffinity column at flow rate of 2 - 3 mL/minute. Column was washed with 20 mL deionized water. Acetonitrile (2.5 mL) was passed through the column and AFM1 was eluted from it. The eluate was dried using water bath at 40 - 50°C in a fume hood. Finally, it was resolved with 1.0 mL of mobile phase. All of the purified extracts were filtered through a 0.20 µm syringe filter (Jet Biofilm company) before injection into the HPLC column.
Stock solution of AFM1 was prepared in acetonitrile at 50 ng/mL. Working standard solutions were prepared from stock standard by diluting with acetonitrile at 0.25 - 1.5 ng/mL. The calibration curve was drawn at concentration of 0.25, 0.5, 0.75, 1.0, 1.25 and 1.5 ng/mL.
The mobile phase was water-acetonitrile-methanol (6:2:2 V/V/V) filtered through a 0.45µm membrane and using flow rate of 1.0 mL/minute.
3.6. Recovery
Recovery percentage was calculated by standard addition method. A certain amount of standard solution with specified concentration (50 ng/mL) was added to the control samples, followed by HPLC analysis according to the material and method section. The recovery was calculated by the following formula and reported as percentage.

4. Results
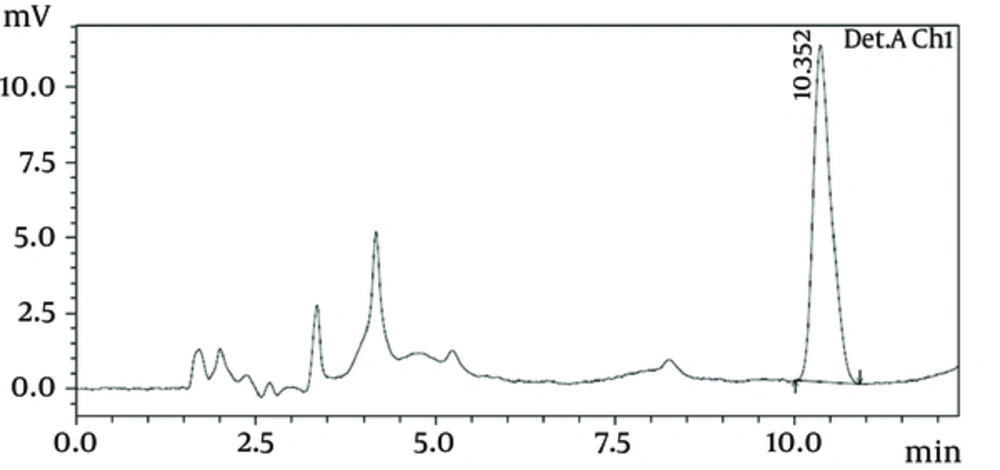
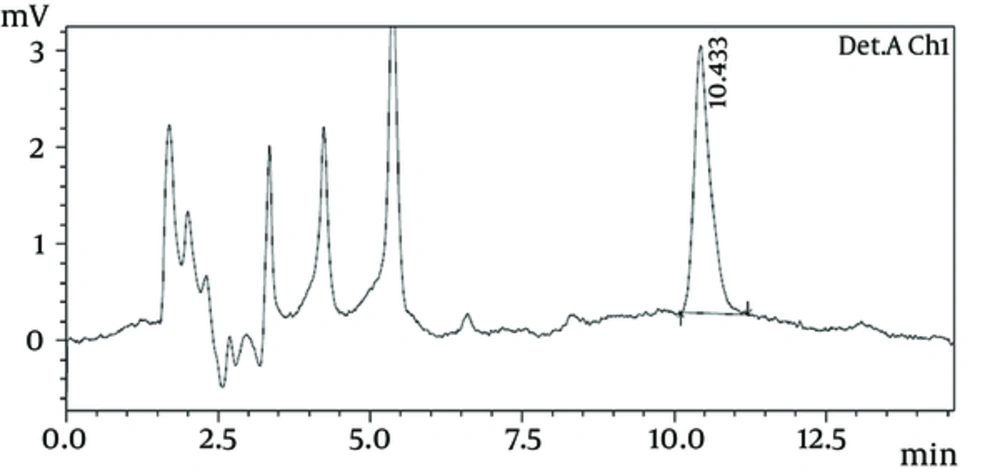
All 117 samples were analyzed by HPLC technique; 9.27% of the raw milk and 5% of the pasteurized milk samples had higher levels of mycotoxins than the maximum recommended limits by FAO. The retention time under test condition was at 10.352 ± 0.152 minute (Figures 1 and 2). The occurrences and levels of AFM1 in raw and pasteurized milk samples are shown in Table 1.
| Type of Milk | N | Minimum | Maximum | Mean |
|---|---|---|---|---|
| Raw | 97 | 0.008 | 0.231 | 0.0238 ± 0.02 |
| Pasteurized | 20 | 0.0024 | 0.131 | 0.03 ± 0.01 |
5. Discussion
Since milk and dairy products are continuously used as an important source of healthy diet by human, especially in infants and children, contamination with AFM1 can be a great risk to human health. There are many methods used to detect the AFM1 in milk and dairy products including enzyme linked immunosorbent assay (ELISA), radioimmunoassay, HPLC with fluorescence detection, biosensors, electrokinetics, electrochemical transduction, amperometric detection and adsorptive stripping voltammetry. Each of these methods has advantages and disadvantages. For example, ELISA has problems such as high background, coefficient of variation, poor standard curve and matrix effect. Nonetheless, the HPLC is the most used quantitative method in research and routine AFM1 detection. This technique is very sensitive but requires skilled operators, extensive pretreatment and expensive equipment.
A few studies have been conducted in Iran and other countries in this context. Galvano et al. determined the level of AFM1 in 161 samples of milk, 92 samples of dry milk and 120 samples of yogurt. The AFM1 was detected in 125 (78%) milk samples (ranging from < 0.001 to 0.0235 µg/kg), 49 (53%) dry milk samples (ranging from < 0.001 to 0.0796 µg/kg) and 73 (61%) yogurt samples (ranging from < 0.001 to 0.0321 µg/kg). Aflatoxin level in only four samples of dry milk was more than legal established level by the European community (EC) in 1999 (10).
In a study conducted by Mohammadi and Alizadeh in Urmia, Iran, the mean of AFM1 in pasteurized and ultrahigh treated temperature (UHT) milk samples were 0.023 and 0.019 µg/kg, respectively, and the level of AFM1 in milk was significantly low (11).
Kamkar et al. examined 122 samples for AFM1 and reported that the mean concentration of AFM1 was 0.04 µg/kg; 14.75% of the samples had higher levels of mycotoxin (9).
Mohammadi Sani et al. measured AFM1 level in milk samples in Khorasan, Iran. The AFM1 level found in all samples had a mean concentration of 0.07792 µg/g. The AFM1 concentrations were lower than those of the Iranian national standard and food and drug administration (FDA) limits, but in 80.6% of the samples these levels were higher than the maximum limit (0.05 µg/kg) accepted by the european union and Codex Alimentarius commission (12).
Shundo and Sabino analyzed 107 samples of raw, pasteurized and UHT milk, and AFM1 was detected in 79 (73.8%) milk samples, ranging from < 0.02 to 0.26 µg/kg (13).
Behfar et al. reported AFM1 in100 samples of pasteurized milk in Ahvaz, Iran, with the mean concentration of 0.0027 µg/kg, which was lower than the maximum accepted level of the country (14).
Mohammadian et al. compared the levels of AFM1 in raw and pasteurized milk samples during different seasons in Sanandaj, Iran. AFM1 was detected in a wide variety of concentrations ranging from 0.000007 to 0.115 µg/kg in 257 (94.49%) out of 272 milk samples. The AFM1 level in 12 (4.4%) mycotoxin contaminated samples were higher than the maximum tolerance limit (0.05 µg/kg) accepted by European Union countries (15).
Rahimi et al. examined 121 dairy product samples consisting of pasteurized milk (60 samples) and white cheese (61 samples), collected from retail markets from August 2010 to September 2011 in Ahvaz, Iran for AFM1. Analytical results showed that 61 (50.4%) samples were contaminated with AFM1 ranging from 0.011 to 0.209 µg/kg (16).
Vagef and Mahmoudi investigated 144 milk samples (102 raw and 42 pasteurized milk samples) in west regions of Iran for AFM1. They found mycotoxin in 43.7% of all samples with the mean level of 0.0394 ± 0.005 µg/kg. The concentration of AFM1 in 21.5% of raw cow milk and 11.9% pasteurized milk samples were higher than maximum tolerance limit accepted by European Union/Codex Alimentarius Commission (0.05 µg/kg) (17).
Sefidgar et al. measured AFM1 in raw milk in Babol and showed that 56.7% of the milk samples were infected to mycotoxin at 0.05 - 0.352 µg/kg (18).
Pournourmohammadi et al. studied the amount of AFM1 in pasteurized milk produced in Kerman city using HPLC with fluorescence detector and demonstrated that the concentration of contamination was below the American standard limit but in 31 samples (44.7%) the contamination was higher than that of the European limit (19).
Considering toxic effects of AFM1 in human, particularly liver cancer and occurrence of AFM1 in dairy products, animal feed should be checked for AFB1 and feed storage should be under appropriate conditions. In addition, milk and its products should be investigated for AFM1 regularly.