1. Background
Burn is a post-traumatic inflammatory disease associated with many local and distant effects resulting in intense inflammation, tissue damage and infection. Increased histamine activity, enhanced by the catalytic properties of xanthine oxidase, causes progressive local increases in vascular permeability. Toxic by-products of xanthine oxidize, including hydrogen peroxide and hydroxyl radical, cause direct damage in dermal structures. Burn initiates systemic inflammatory reactions by producing toxic mediators such as reactive oxygen species (ROS), almost in every tissue directly or indirectly (1). Oxygen radicals are involved in the inflammatory process following thermal injury. Oxygen radicals participate in some pathophysiological processes such as formation of edema into zone of stasis. Three zones of burns are: coagulation, stasis and hyperaemia. Any additional results such as oedema can convert stasis zone into an area of complete tissue loss that leads to wound deepening (2). Clinical response to burn is dependent on the balance between production of free radicals and its detoxification. Production of ROS is physiologically regulated in cells (1). Normally, to prevent the destructive potential of oxygen radicals, cells are able to defend by preventing or limiting oxidative injury. These cyto-protective mechanisms, known as antioxidant defenses, include several enzyme systems designed to scavenge oxygen radicals and detoxify them. They exist in both the aqueous and membrane compartments of cells and can be either enzymatic or non-enzymatic antioxidants (3). One of the enzymatic defenses against ROS is superoxide dismutase (SOD), which converts the superoxide radical to less bioactive hydrogen peroxide and oxygen molecules and plays an important role in self-defense mechanisms of cells against oxidative stress. The delicate balance between antioxidants and oxidant production during burns and many pathophysiological conditions may be disrupted by either deficient antioxidants or excess oxidants. Exogenously taking antioxidants such as SOD can partly decrease burn injury (1).
Superoxide dismutase is a metalloenzyme, which is found at high levels in eukaryotic aerobic cells under normal physiological conditions (4). Some clinical uses of this enzyme include rheumatoid arthritis, aging, cancer and respiratory distress syndrome (5). Superoxide dismutase has been investigated for use in the treatment of several diseases in which the superoxide radical is involved (6). Treatment using SOD appears to be a promising alternative to conventional therapy. Various studies have attempted to use it in the treatment of oxidative stress-related diseases such as burns (7). These studies were mainly based on systemic application of SOD. However, because of its short biological half-life, relatively high molecular weight and hydrophilic nature, the tissue protecting effect of systemically administered SOD has been reported minimum, and hence repeated administration for achieving the therapeutic effect is required (8). Additionally, the SOD becomes inactivated by its own reaction product, hydrogen peroxide, also generating very toxic radical species in the organism (9).
Major approaches have been attempted to avoid these problems with the clinical application of SOD. One involves improving SOD properties by chemical modification through covalent linkage to hydrophilic molecules (9). The other is by increase in therapeutic effectiveness of enzymes through using controlled-release systems composed of hydrophilic polymer hydrogels of proven biocompatibility (9). Topical and transdermal drug delivery such as liposomal formulation was also used for SOD delivery (6).
Solid lipid nanoparticles (SLNs), a new nanoparticle-based drug delivery system with range in diameter from 10 to 1000 nm has attracted significant attention. The advantages of SLNs compared to conventional drug delivery systems include improved efficacy, reduced toxicity, protected active compounds and enhanced biocompatibility (10).
Solid lipid nanoparticles are very attractive colloidal carrier systems for skin applications due to their various desirable effects on skin besides the characteristics of a colloidal carrier system. They are well intended for use on damaged or inflamed skin because they are prepared using non-irritant and non-toxic lipids (11). General features of SLN are their composition of physiological compounds, possible routes of administration such as intravenous, oral and topical, and the relatively low costs of excipients. The other advantage is easy large-scale production (12).
It has been reported that under optimized conditions they can be used to incorporate hydrophobic or hydrophilic drugs. Formulation in SLN improves protein stability, avoids proteolytic degradation, as well as sustained release of the incorporated molecules (11).
2. Objectives
Considering the features mentioned above, in order to deliver SOD topically and enhance permeation through burned rat skin as well as protecting it from environmental degradation, it was aimed to prepare SLN loaded with SOD.
3. Methods
Superoxide dismutase (S 2515, 15000 U, Lot 068K 23654 from bovine Erythrocytes, 2500 - 7000 Unit/mg Proteins), SOD assay Kit (19160-1KT-F, Lot N. BCBF6457), and Bradford Reagent were purchased from Sigma-Aldrich; Compritol®888 ATO (Glycerylbehenate) was a gift from Gattefosse Pharmaceuticals (France), Soybean Lecithin from Serva Feinbiomedica (Heidelberg/ New York, USA), Span 20 and Tween 80 from Daejung Chemicals and Metals Co. (Korea). Oleic acid, propylene glycol and polyethylene glycol 300 were provided from Merck. Deionized double distilled (DDD) water was used wherever required.
3.1. Factorial Experimental Design
Eight formulations were prepared using factorial design with different amounts of ingredients. Table 1 shows the variables with their levels. The levels were based on preformulation studies.
| Variable | Up Level | Low Level |
|---|---|---|
| Lipid phase percent | 90% | 70% |
| Liquid lipid/solid lipid | 15: 85 | 5: 95 |
| Surfactant | Tween 80 + Span 20 (1: 1) | Lecithin |
3.2. Preparation of Solid Lipid Nanoparticles Containing Superoxide Dismutase
Cold homogenization technique was applied to prepare solid lipid nanoparticles loaded with SOD. Solid lipid (Compritol) was indirectly heated (up to 65°C) to be melted and then liquid lipid (oleic acid) was added and mixed. Next, surfactant either lecithin or mixture of Tween 80 and Span 20 in proportion of 1: 1 and SOD was dissolved in water and was added to melted lipid phase and stirred until a homogenous mixture was formed, and was then sonicated (ELMA, S 30H, Elmasonic, Germany) at 90 w and 37°C for two minutes, and congealed by adding a mixture of water and propylene glycol at 4°C (in proportion of 4: 1) to reach 60 mL of volume. The mixture was contemporaneously homogenized using high speed homogenizer (IKA® T25 digital ULTRA-TORRAX®, Germany) at 12000 RPM for 20 minutes (11, 13).
3.3. Enzyme Assay Method
Activity of SOD was determined with a kit based on ability of enzyme to convert superoxide radicals, produced by xanthine/xanthine oxidase, to hydrogen peroxide and to inhibit indicator oxidation and color formation (6, 7, 14).
In order to calculate the entrapment efficiency and drug release profiles, protein concentration was determined using the Bradford Method, a general method for quantification of total protein using BSA as a standard protein.
3.4. Measurement of Particle Size
After diluting formulations, the size and size distribution of particles were analyzed using Particle Size Analyzer instrument (Scatteroscope 1 Qudix, Korea). Each sample was assessed three times and the average of results was calculated (15).
3.5. Differential Scanning Calorimetry (DSC) Studies
To study the behavior of nanoparticles under thermal stress, differential scanning calorimetry (Mettler Toledo, Switzerland) was used. The effect of independent variables on phase transitional temperature, enthalpy of each peak, interaction between ingredients and SOD were studied through heating and cooling programs. An empty aluminum pan was used as the reference (10).
3.6. Determination of Entrapment Efficiency
To calculate the entrapment efficiency (EE %) of particles, 2 mL of each formulation was centrifuged (Vision, Scientific Co. LTD, Korea) at speed of 30000 RPM for 25 minutes at 4°C to separate the unloaded drug. The particles were rinsed twice with water and added to the supernatant to separate the unloaded drug completely. This part was used to determine the un-entrapped drug using the following equation:
EE% = (Total drug-un entrapped drug) × 100 / Total drug
On the other hand, the SLN-encapsulated SOD activity and amount of protein were measured after SLNs were treated with Triton X-100, as detergent with concentration of 0.5% (w/v). The effect of Triton X-100 on SOD activity was evaluated by using SOD-standard dissolved in the detergent solution (6).
3.7. In Vitro Release of SOD
Apparatus No. 2 USP of dissolution was used for the drug release study. For each formulation, 10 mL was centrifuged at speed of 30000 RPM for 25 minutes at 4ͦ C to separate particles. The particles were washed twice with water and then suspended in 50 mL of phosphate buffer, pH = 7.4. The suspension was poured in apparatus vessel and speed and temperature were adjusted on 50RPM and 37°C, respectively. At intervals of 0.5, 1, 2, 3, 4,6, 8, 12, 24, 36 and 48 hours from the beginning of the experiment, sampling was carried out by withdrawal of 2 mL of each formulation and replaced with 2 mL of fresh phosphate buffer to maintain the volume constant. The cumulative percentage of drug released was illustrated against time.
3.8. Study of Permeation of Solid Lipid Nanoparticles Through Burned Rat Skin
Eight male Vistar rats with average weight of 226 ± 22.1g were kept for seven days of acclimatization, and given a standard diet and water. Water and food were removed one hour before experimental trauma. The animals were anaesthetized by intraperitoneal injection of ketamine/xylazine, shaved, and burned dorsally by a smooth metal surface preheated to 100°C by electrical power for 15 seconds to produce a uniform deep second-degree burn with a size of 4 cm in diameter. Afterwards, the animals were killed with excess dose of ketamine, and burned skin was removed and after cleaning the additive lipids using acetone, mounted on static Franz cell between donor and receiver compartments. The donor medium was 2.5 mL of SLN loaded SOD and a SOD solution in buffer phosphate (pH 7.4) with the same concentration as control and the receiver contained phosphate buffer (pH = 7.4). The temperature and stirring of medium were adjusted to 37°C and 200 RPM, respectively. Sampling at intervals of 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, and 72 hours was carried out by withdrawal of 2 mL from receiver part and replacement with 2 mL of buffer. The amount of drug permeated was determined and the cumulated amount of drug was plotted against time (8). The animals were treated according to the principal for care and use of laboratory animals and approved by the ethical committee of Ahvaz Jundishapur University of Medical Sciences.
3.9. Study of Solid Lipid Nanoparticles Stability
All formulations were stored at 2 - 8°C for six months and particle size, drug content, appearance and drug release were assessed. The results were correspondingly compared with the values of freshly prepared samples (11).
3.10. Statistical Methods
The one-way analysis of variance (ANOVA) statistical test and paired T-test were used to compare formulations. In addition, to study the relationship between variables and responses, concurrent multiple regressions were carried out. P value of 0.05 and less was considered as significant correlation when needed.
4. Results
4.1. Solid Lipid Nanoparticles Characterization
The characteristics of SLNs prepared by cold homogenization method and based on factorial experimental design is shown in Table 2.
| Formulation No. | Factorial State | Percentage Lipid Phase | Percentage Surfactant | SOD Solution, 5 KU/mL, mL | Mean Diameter, nm | Percentage Entrapment Efficiency | Percentage Activity, (%) |
|---|---|---|---|---|---|---|---|
| 1 | +++ | 90 | 5 | 0.2 | 37.1 ± 5.20 | 85.40 ± 9.33 | 28.5 ± 3.35 |
| 2 | ++- | 90 | 5 | 0.2 | 17.98 ± 5.47 | 68.20 ± 5.12 | 61.5 ± 4.18 |
| 3 | +-+ | 90 | 5 | 0.2 | 21.3 ± 0.80 | 92.70 ± 6.82 | 41.8 ± 4.53 |
| 4 | +-- | 90 | 5 | 0.2 | 18.4 ± 2.00 | 79.17 ± 5.35 | 28.2 ± 1.55 |
| 5 | -++ | 70 | 5 | 0.2 | 34.05 ± 17.15 | 89.70 ± 9.11 | 27.1 ± 2.1 |
| 6 | -+- | 70 | 5 | 0.2 | 70.22 ± 16.88 | 73.43 ± 6.63 | 36.3 ± 2.24 |
| 7 | --+ | 70 | 5 | 0.2 | 29.05 ± 14.55 | 90.10 ± 7.76 | 54.5 ± 3.44 |
| 8 | -- | 70 | 5 | 0.2 | 102.2 ± 4.8 | 78.05 ± 8.25 | 24.8± 1.95 |
4.1.1. Particle Size
Table 2 shows particle size of formulations and as seen, the smallest and largest sizes for freshly prepared samples belong to formulation No 2 and No 8, respectively. Formulation No. 2 contained high level of lipid and LL/SL proportion, yet formulation No. 8 consisted of low level of total lipid, and low level of LL/SL ratio. All formulations, except one, had a size less than 100 nm. Regression analysis of results showed no significant correlation between independent variables and particle size (P > 0.05). In contrary to our findings, Shi et al. (10), reported smaller SLN with higher amount of lipid, surfactant/lipid ratio and high pressure hot homogenization preparation method, for frankincense and myrrh essential oils as lipophilic compounds. It seems that combination of compensating factors and hydrophilic nature of SOD causes no significant correlation in this study. Therefore, to reach a significant correlation between variables and particle size, the levels of variables should be modified. In contrast, a narrow particle size distribution was observed in all formulations. The particle size growth was seen after six months that may be due to high content of solid lipid and low concentration of surfactant.
4.1.2. Entrapment Efficiency (EE)
Solid lipid nanoparticles demonstrated enzyme loading between 68 and 93% that is the optimum value for hydrophilic compounds such as SOD. Loading amount was affected by LL/SL ratio (P = 0.04) and type of surfactant (P = 0.041). Both independent variables showed significant and indirect correlation with entrapment efficiency. Therefore, higher amount of liquid lipid and using of lecithin as surfactant caused higher enzyme entrapment that is in accordance with the hydrophilic nature of SOD. Although percentage of lipid didn’t show any significant correlation with EE, yet using a suitable amount of liquid oil and surfactant especially lecithin increased enzyme loading. Different vehicles such as Poly (DL-Lactide-Co-Glycolide) (PLGA) microsphere (16, 17), alginate-chitosan microsphere (16), cationic liposome (18) and mucoadhesive chitosan-coated liposome (7) have been used as SOD drug delivery systems. The highest and lowest SOD entrapment efficiency were 92% and 17%, which were provided by alginate-chitosan microspheres and cationic liposomes, respectively. Entrapment efficiency in chitosan-coated liposome (64%) was more than cationic liposomes (17%). It seems that solubility, particle size and SOD-excipients interaction play critical roles in the amount of entrapment efficiency. In the present study, SLNs demonstrated EE equal to alginate-chitosan microspheres and chitosan-coated liposomes. Therefore, oleic acid and lecithin used as liquid oil and surfactant solublized SOD and produced high EE percentage. On the other hand, SLNs with low particle size caused high area and high enzyme loading. Solid lipid nanoparticles that were produced in the present study had smaller particle size than other delivery systems mentioned above.
4.1.3. Activity
Solid lipid nanoparticles are activated immediately after preparation, as illustrated in Table 2. Highest and lowest activities were 61.5% and 24.8% that were produced by formulation 2 and 8, respectively. Although there is no significant correlation between EE percentage and activity yet it can be considered that high EE percentage is the reason for low activity immediately after SLN preparation. Likewise, no significant correlation was found between activity and independent variables. This means that activity is an independent variable and shows interaction between enzyme and substrate. This result is in agreement with other findings that reported SOD loading on chitosan microspheres (6) and liposome (18). In order to determine the effect of preparation method on enzyme activity, a recovery test was performed. The recovered SOD activity was 85.3% of initial SOD activity that is lower than that reported in literature (7). Therefore, it can be concluded that there was little activity loss during the production procedure. The effect of Triton X-100 on SOD activity was evaluated and results showed that Triton decreased enzyme activity to less that 5%. Therefore in conclusion, preparation method and Triton X-100 decreased lower %15 that implies enzyme protection.
4.2 Differential Scanning Calorimetry Analysis
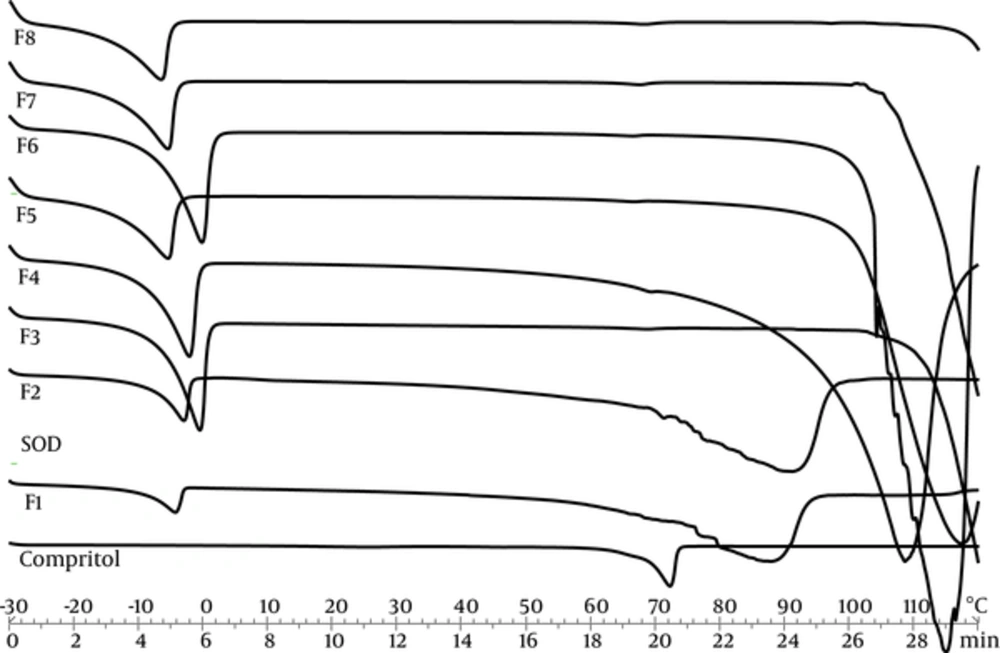
Differential scanning calorimetry can be used to determine thermodynamic differences related to morphological changes and crystallinity, because each lipid has its own melting point and melting enthalpy. In this research, DSC was performed to study the melting and crystallization behavior of the lipid matrices to determine whether these characteristics were changed by other ingredients and SLN’s properties. Figure 1 represents thermal curves for all formulations of SOD-loaded SLNs, SOD alone as the active ingredient, and SLN without SOD as control, through heat scanning program.
Temperature and enthalpy of transitions that appeared in heating thermo grams are shown in Table 3. Three transitions appeared with the first and third transitions describing water melting and boiling points, respectively. The second transition indicates lipid melting point. Comperitol demonstrated that the melting point at 72°C was the same as that reported by Aburahma et al. (19). The control SLNs and SOD loaded SLNs presented a lower melting point and enthalpy. This reduction is due to Nano crystalline size of lipid in SLN dispersions (20). The SOD didn’t present transition in this temperature. The crystallinity index percentage (CI %) of SLNs was calculated by the following Equation 1 (21):
| Formulation | Heating | |||||
|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | ||||
| T (ᵒC) | ∆H | T (°C) | ∆H | T (°C) | ∆H | |
| F1 | -4 ± 0.5 | -31.7 ± 1.25 | --- | --- | 88 ± 5 | -346.59 ± 25.4 |
| F2 | -3 ± 0.2 | -40.44 ± 2.37 | --- | --- | 91 ± 6 | -360.24 ± 33.51 |
| F3 | 0 ± 0.1 | -194.45 ± 10.19 | 68 ± 3 | -1.4±0.3 | 102 ± 4 | -9.89 ± 2.63 |
| F4 | -2 ± 0.2 | -130.74 ± 8.35 | 69.5 ± 4.4 | -1.9±0.5 | 109 ± 7 | -1098.3 ± 41.21 |
| F5 | -5 ± 0.3 | -90.79 ± 5.62 | 67 ± 5 | -0.65±0.15 | 117 ± 9 | --- |
| F6 | 0 ± 0.2 | -164.2 ± 12.38 | 67 ± 4 | -0.73±0.22 | 115 ± 4 | -1458.8 ± 64.47 |
| F7 | -5 ± 0.3 | -105.4 | 68 | -2.52± | 120 | --- |
| F8 | -6 ± 0.2 | -115.59 ± 7.81 | 69 ± 5 | -1.8±0.55 | 125 ± 14 | --- |

A high value of the melting enthalpy describes a high level of organization in the crystal lattice. Therefore, a less ordered structure was obtained by SLN formation. All formulations demonstrated CI% below 30%. Hence, low value of CI% leads to slower drug release and means that lower energy is required to melt the crystal lipids (22). Therefore, low amount of CI% is sufficiently low to ensure the sustained release of SOD, yet isn’t sufficiently high to make sure SLN stability. In this study, the significant and direct correlation between CI% and particle size were seen.
4.3. Release Profile
Amounts of SOD released from all formulations within different times are presented in Table 4. As seen, the minimum amounts belonged to formulation No. 1 except on the 48th hour. F8 represented the maximum amounts at all-time points.
| Time (H) | % SOD Released | |||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
| 1 | 11.20 ± 0.9 | 24.15 ± 2.2 | 15.53 ± 1.4 | 17.63 ± 1.1 | 23.47 ± 1.6 | 38.45 ± 1.4 | 16.30 | 40.95 |
| 2 | 16.68 ± 1.1 | 25.93 ± 1.6 | 18.56 ± 1.3 | 20.74 ± 0.8 | 24.05 ± 2.3 | 39.55 ± 2.2 | 17.78 ± 1.3 | 43.284.5 |
| 4 | 18.58 ± 1.3 | 26.77 ± 1.1 | 20.28 ± 0.9 | 22.25 ± 1.9 | 27.75 ± 1.3 | 42.98 ± 3.9 | 18.95 ± 1.7 | 47.12 ± 5.1 |
| 6 | 19.79 ± 0.9 | 28.93 ± 2.3 | 22.64 ± 2.1 | 24.76 ± 1.2 | 29.82 ± 2.2 | 45.69 ± 2.7 | 22.73 ± 1.5 | 51.33 ± 3.7 |
| 8 | 22.57 ± 1.5 | 31.82 ± 2.1 | 24.91 ± 1.8 | 27.11 ± 2.4 | 32.56 ± 1.1 | 49.34 ± 2.5 | 26.10 ± .5 | 54.42 ± 4.1 |
| 24 | 26.97 ± 1.9 | 36.79 ± 3.2 | 28.36 ± 1.3 | 31.18 ± 1.8 | 35.42 ± 1.5 | 56.17 ± 4.3 | 30.21 ± 2.7 | 60.92 ± 4.9 |
| 48 | 31.47 ± 2.6 | 41.95 ± 2.3 | 30.75 ± 2.5 | 35.24 ± 1.5 | 38.88 ± 2.5 | 59.55 ± 3.6 | 34.83 ± 3.2 | 65.18 ± 2.9 |
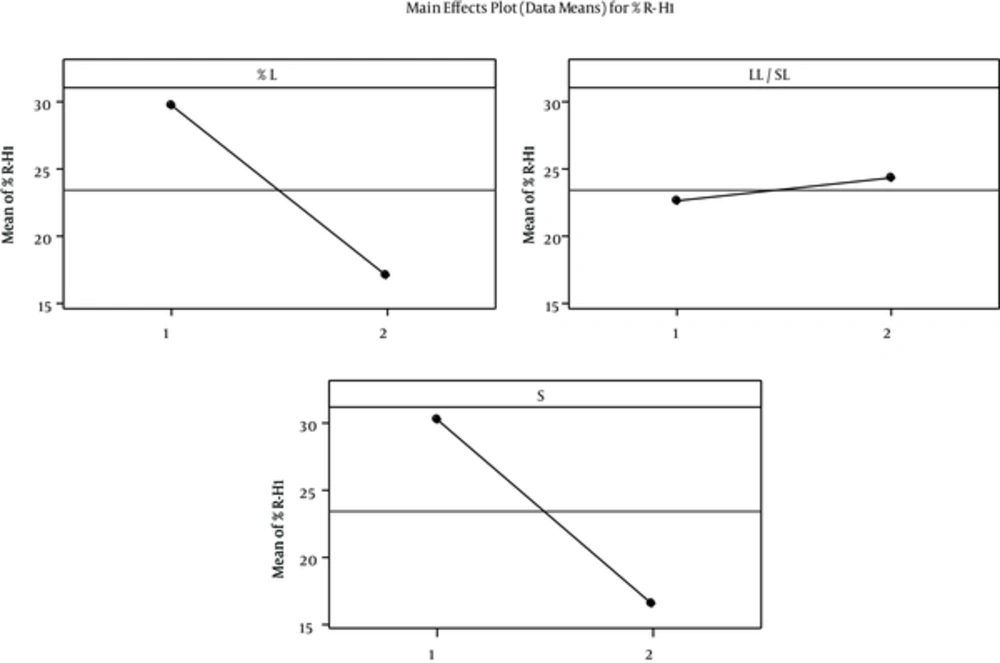
The percentage of SOD released after 1 (R-H1%) and 48 hours (R-H48%) was selected as a sign of fast and delayed release. Regression analysis of results showed a significant correlation between independent factors L & S% and R- H1% with p values of 0.034 and 0.027, respectively. Increase in the L% and changing the surfactant from “Tween80 + Span20” to Lecithin reduced the percentage release. The correlation between R-H1% and independent variables is illustrated in Figure 2.
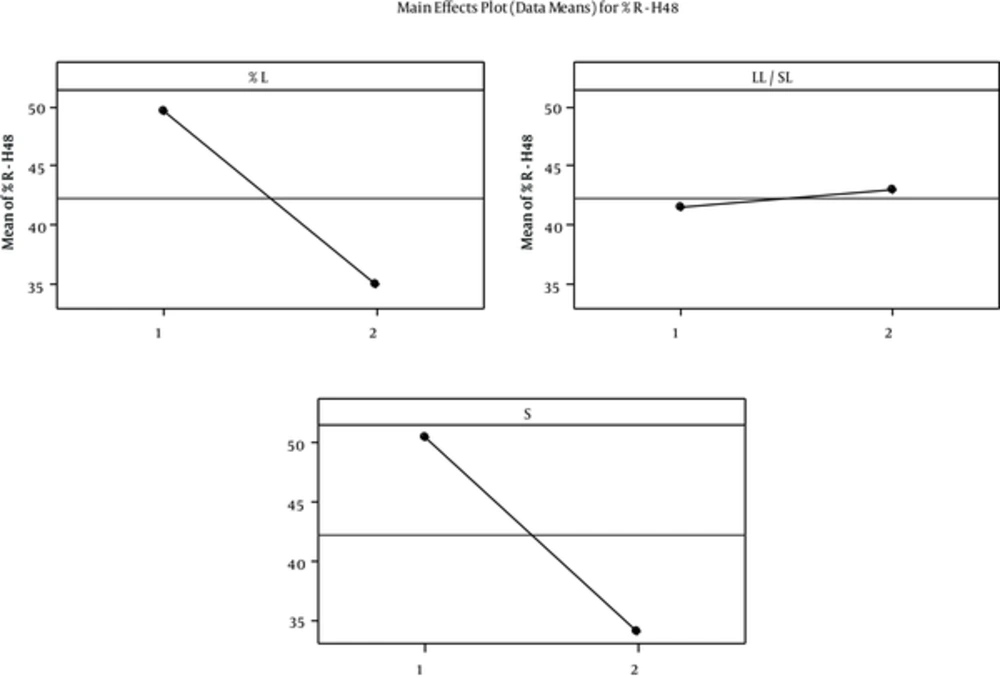
Furthermore, L & S% had a significant and indirect effect on R-H48% that is shown in figure 3. Release pattern suggests burst release with all formulation especially No. 8, which means SOD loaded on the surface of SLNs that is 11% - 41% of total loaded enzyme released fast . However, some parts of SOD that were incorporated in the core have sustained release. This finding indicated core-shell incorporation model for SOD loaded in SLNs. This model provides good property for fast and sustained SOD delivery through burnt skin. Different mathematical models used for 60% of data aim to determine the kinetic of SOD release from SLNs. We found that first order Higuchi and Hixson were the best models for SOD release data. This means that diffusion or dissolution controls the SOD release rate and geometrical shape keeps constant during the release experiment (23). However, validity of first order and higuchi models indicated SOD was dispersed uniformly in this manner that there is a concentration gradient from shell towards core. This incorporation model is in accordance with aqueous solubility of SOD. Low enzyme released after two days suggests SOD suspended in the SLN and its solubility in SLN limited percentage release. Celik et al. (6) reported SOD burst release from chitosan microspheres; 70-80% of enzyme was released from microspheres within first day and release was completed during four to eight days. The SOD burst release from PLGA and chitosan-alginate microspheres was 18% and 50% after 24 hours (16).
4.4. Superoxide Dismutase Activity in the Release Experiment
Percentage activity of enzyme at 1, 8 and 24 hours in release medium was assessed. The results are shown in Table 5. These times were selected as a sign of fast, intermediate and sustained enzyme release and their activity in release medium.
| Formulation | Percentage Activity | ||
|---|---|---|---|
| H1 | H8 | H24 | |
| F1 | 25.3 ± 2.33 | 55.74 ± 3.94 | 60.79 ± 5.33 |
| F2 | 29.83 ± 3.12 | 60.67 ± 4.71 | 64.73 ± 4.84 |
| F3 | 26.96 ± 1.86 | 62.39 ± 5.43 | 68.45 |
| F4 | 21.84 ± 1.55 | 48.93 ± 4.60 | 59.17 ± 4.74 |
| F5 | 12.42 ± 1.1 | 37.52 ± 2.29 | 51.54 ± 5.36 |
| F6 | 20.96 ± 1.77 | 46.55 ± 1.94 | 57.50 ± 5.23 |
| F7 | 18.75 ± 2.16 | 45.28 ± 3.82 | 58.82 ± 4.79 |
| F8 | 15.30 ± 1.24 | 39.95 ± 4.05 | 50.77 ± 3.51 |
There was a significant and direct correlation between percentage lipid and percentage activity at one (P = 0.039) and eight (P = 0.024) hours and no significant relationship with percentage activity after 24 hours. The highest and lowest percentage activity were presented by formulation No. 2 and 5, respectively. This finding indicated the same pattern in percentage activity at all times that activity was measured. Based on these results we can conclude that percentage of enzyme released and activity were mainly controlled by percentage L.
However, there were no significant correlations between independent factors and percentage activity at 24 hours.
4.5. Superoxide Dismutase Permeation Through Burned Rat Skin
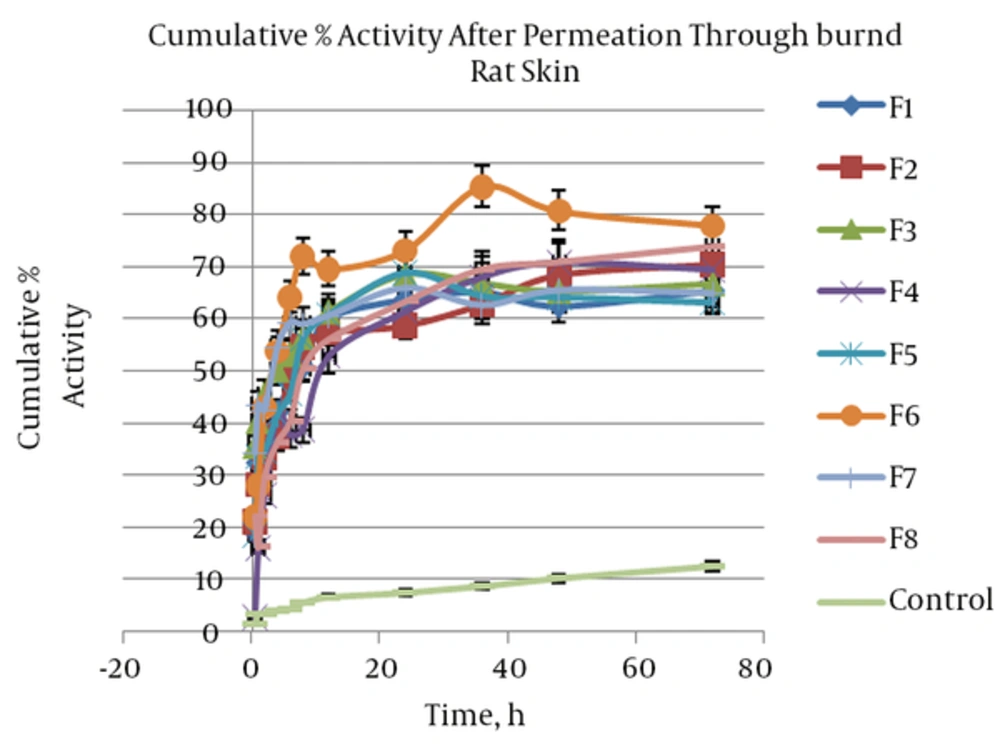
The cumulative percentage activity of SOD that was measured after permeation through burned rat skin was shown in Figure 4. Percentage activity of enzyme at 1, 8, and 72 hours after permeation through burned rat skin a sign as fast, intermediate and sustained enzyme permeated and their activities was assessed.
Based on these results, percentage activity after one hour was between 15 and 44% and was 5 to 13 folds more than the control. Analysis of regression proved significant (P = 0.04) and direct correlation between independent variables and percentage activity after one hour (H1). Therefore, higher H1 was provided by a formulation including tween and span as a surfactant blend. Likewise, significant (P = 0.028) but indirect correlation was found about percentage activity after 72 hours (H72). This means lecithin as a surfactant increased H72. At other times no significant correlation was seen between percentage activity and surfactant type and other independent variables. We are facing surfactant binary behavior. In the initial hours of skin permeation, SOD permeated through burned rat skin, increased when SLN was made by tween and span, and after 36 hours higher SOD permeation was provided by SLN including lecithin. It seems that lecithin caused more SLN fusion in the skin that brings about higher enzyme release and permeation through rat skin. Therefore, it may be that this effect of lecithin needs time more than 36 hours. In conclusion all formulations induced high enzyme activity in burned skin during 72 hours after topical application, mostly when lecithin is applied as surfactant in SLNs. After 72 hours, percentage activity provided by SLNs dispersion was 5 - 6.5 folds more than the control. This finding suggests SLN improved SOD partitioning into skin and increased SOD solubility in skin as a lipophilic biological membrane. Kigasawa et al. (18) developed a non-invasive Trans follicular delivery for SOD with a combination of cationic liposomes and iontophoresis. They reported that SOD-liposome could not penetrate at the skin and hair follicles. They believed that penetration of liposomes with a diameter greater than 100 nm through the stratum corneum is difficult due to the narrow intracellular space. The same results were reported about insulin-encapsulated liposomes (24). Also Lasch et al. (25) indicated fluorescent-labeled lipid of liposomes applied over the stratum corneum and didn’t penetrate in the skin after 24 hours. In comparison with liposome SLNs that were prepared in the present study, penetration into the skin was found due to particle size smaller than 100 nm. Therefore, SLN dispersion can be a proper vehicle to pass SOD through burn rat skin. The SLN corrected SOD properties and increased its partitioning into the skin and then diffusion through intracellular space. Moreover, SLN protected SOD and induced high percentage of activity of enzyme across the burned skin.
4.6. Stability
Particle size, drug content and dispersion appearance were evaluated after six months as signs of stability. Results showed after six months particle size increased from 20 - 120 nm to 100 - 150 nm. This means that particle size increased more than two folds. Particle size increase indicates that there is a risk of particles aggregation due to low CI percentage and a less ordered structure that appeared during storage. However, after six months, enzyme content and activity and dispersion appearance didn’t change. Therefore, particle size increase didn’t cause instability.
5. Discussion
In this study, SOD encapsulated into SLNs with 60% - 90% entrapment efficiency. Beside high percentage of lipid, using of lecithin as a surfactant increased SOD loading. Low lipid crystallinity index and low crystal lattice energy helped greater SOD entrapping into the SLNs. Proper selection of lipids and other excipients and their amounts increased SOD loading and enzyme protection against environmental degradation. The results indicated that SOD-encapsulated in SLN could be delivered into the deep burned skin layer and induce high enzyme activity everywhere on the skin. Low particle size, application of lecithin as surfactant, and low CI percentage, were important factors for increasing SOD penetration through the burned rat skin. A small particle size growth occurred during storage, which didn’t change SLN dispersion appearance and stability.