1. Background
Nowadays, medicinal plants and their use for treatment of some diseases have drawn the attention of people worldwide. In fact, they store active components in the organelles named vacuole, which similarly act as human liver so that some active ingredients used for therapeutic aims are located in these organelles (1). Among the plant families, the plants from Cruciferae family are of importance because of having protective properties such as anticancer, antigenotoxic, antioxidants, antihepatotoxic, antihypercholesteremia, and a series of the other properties that have been attributed to some of the plants presented in this family (2-7).
In Iran, because of having various climatological conditions, a broad spectrum of medicinal plants with different properties is available. It is obvious that the meteorological parameters can affect the plant properties and finally this matter can produce the interchangeable toxic and pharmacologic effects (8). Some of the cruciferous plants have been reported that, if taken as a prophylactic procedure, will be able to prevent developments of some cancers in human (9). Thus, the cruciferous plants are consumed noticeably. If deeply being looked over the properties of cruciferous plants, we will find out that the presence of some compounds namely glucosinolates (GLS) that can be found remarkably in some plants of this family like cabbages may cause to have a protective role against some malignant cancers such as breast cancer (10).
The putative mechanisms considered for the protective action of these plants are due to the enzymatic breakdown of GLS compounds and their further conversion into isothiocyanates that cause them to be more potent (11). By this action, cruciferous plants will be able to counteract against cancers and consequently, they can be targeted in therapeutic strategies for development of novel drug alternatives based on herbal and natural resources used against carcinogenesis (12). In fact, the GLS compounds that are present remarkably in plants of this family, after being metabolized, undergo a series of changes and as a result, they will convert into other bioactive compounds named isothiocyanates (13). The latter compounds are believed to be very potent and toxic to growing cancer cells and therefore, they can be efficient and useful in the treatment of cancers.
Our previous and preliminary studies on four cruciferous plants including Cardaria draba (CD), Rorippa sylvestris (RS), Sameraria nummularia (SN), and Cardamine uliginosa (CU) showed that they are able to remove free radicals in test tubes. Additionally, some of them have shown high antioxidant capacity when compared with potent reducing agents such as ascorbic acid (4, 14). Little is known about the toxicity or protective effects of these plants while most of them are grown in our district namely Alborz province as wild plants (15). Among these plants, the plant SN holds a high amount of GLS and has been reported to have scavenging activities (7). CU contains an outstanding glucosinolate named gluconasturtin (GNST), which can undergo hydrolysis to form phenylethylensenevol. GNST is believed to have a prominent role in the genotoxic responses (16, 17). Some of these plants such as CD and RS grow near the river and have strong vegetative production. CD is a plant that is named in Persian as Ozmak. In Iran, it can be used as a vegetable found close to the farm where water flows around its ecological niche. There has been reported that CD seeds contain a high amount of toxic compounds that are noxious to domestic animals. However, brewing the leaves of this plant makes it non - toxic and in this way, it would able to inhibit inflammations and inflammatory mediates in the respiratory system (18, 19).
Nowadays, global efforts have been focusing on finding anticancer drugs with plant - based derivatives like gallic acid (GA). In this regard, our preference is to find some compounds with bioactive components and with noticeable toxicity so that they can be used for their anticancer effects (20). Among cruciferous plants, there are many plants with unknown toxicities and little is known about their protective effects. In addition, studies carried out on this family plant have been mostly concentrated on few plants of this family, especially some kinds of cabbages, synapses, and cresses. Therefore, the aforementioned plants merit further studies to evaluate their bioactive compounds through toxicity studies.
2. Objectives
The aim of this study was to evaluate the 24 h and 48 h toxic effects of 4 cruciferous plants using the brine shrimp lethality assay that is a proper and useful indicator for determining cytotoxicity of medicinal plants and their safety. The results of this study can enable us to isolate bioactive compounds from the extract(s) of those that showed notable toxicity against brine shrimp in comparison with naturally based drugs.
3. Methods
3.1. Chemicals
Dimethyl sulfoxide (DMSO) was obtained from Samchun Chemicals Company (Pyeongtaek, South Korea). Vincristine sulfate was purchased from Sobhan Oncology Company (Rasht, Iran), which was the official representative of Gedeon Richter pharmaceutical company (Budapest, Hungary). Ethanol was purchased from Razi Chemical Company (Tehran, Iran). 2,2 - Diphenyl - 1 - picrylhydrazil (DPPH) was supplied by Sigma Chemicals Company (St. Louis, MO, USA). All the chemicals used for this study had the highest purity (> 99%).
3.2. Plant Materials
The plant samples were collected from the northern part of Iran in Alborz province at an altitude of 1400 meters above the sea level. The plants were identified at Herbarium of Imam Khomeini Higher Education Center (IHEC) and the vouchers specimens were kept at the Medicinal plants Department of IHEC. The plants were then placed in boiled alcohol for 5 minutes and dried under shade.
3.3. Extraction
The whole parts of each plant were distinctly powdered using mortar and pestle. The powdered plants were macerated in ethanol 80% for 3 times of 48 h. The alcoholic extracts of each plant were then concentrated by an evaporator system under reduced pressure and temperature below 40 ºC. The total extracts obtained from this step were further used for bioassay tests of brine shrimp.
3.4. Toxicity Bioassay Tests
Artemia salina (brine shrimp) eggs were hatched in artificial seawater. Artificial seawater was made by dissolving 38 g of salt in 1 liter of distilled water. After 48 h incubation (22 - 28ºC), the larvae (nauplii) were hatched from eggs and attracted to a light source in a conical flask. The larvae were separated from the eggs by pipette and collected in a beaker.
The brine shrimp lethality bioassay technique in accordance with Meyer’s method (21) was used for determination of toxicity of four plant extractives. First, the plant extracts (128 and 160 mg) were dissolved in 10 mL of dimethylsulfoxide (DMSO) and diluted with artificial seawater so that the consequence concentration of DMSO in the prepared stock solution did not exceed 0.05%. Then, DMSO solutions of the extracts were placed in 12-well microplates that contained 10 larvae of Artemia salina. The plant extracts (0.5 mL) were placed on each plate and made up to 5 mL with a brine solution. The final concentrations of the extracts through serial dilution were in the range of 40 to 800 μg/mL. A row in microplate was left as control experiment consisting of 5 mL of brine solution, DMSO, and 10 nauplii. All microplates were then incubated at temperature 24ºC for 24 h and 48 h periods under light conditions. Then, the microplates were examined for the number of dead larvae (nauplii) using a microscope that was set to a magnification of 100.
The toxicity of each extract against brine shrimp was determined by taking an average of three experiments using probit analysis method (8, 21). Briefly, log concentration, total number, and the dead number of shrimps and accordingly their mortalities were calculated. Then, the corrected mortalities were obtained by using Abbott’s formula and finally, after probit transformation, the LC50 values of the extracts were calculated by regression methods. The LC50 values were again measured and confirmed by the Pharm/PCS software. Chi (X2) test was also used for showing any difference between observed and expected mortality.
3.5. Determination of Scavenging Activities of Four Extractives
Anti - free radical scavenging activities of the four extracts of CD, RS, SN, and CU were determined using free radical DPPH based on the methods that adopted for antioxidant activities of plant extracts (4, 7, 14). Briefly, different concentrations of each extract were added to a chemical reaction that was included with 0.1 mM of DPPH radical and the reduction of its absorbance was spectrophotometrically read at 517 nm.
3.6. Association between Cytotoxicity Properties and Free Radical Scavenging Activities in Four Plant Extracts
The correlation between cytotoxicity property and scavenging activity of four cruciferous plants was made by drawing a regression line resulted from inhibition percentage of DPPH free radicals in test tubes for each plant extract and the corresponding cytotoxicity obtained from the extracts against brine shrimp. The correlation coefficient (R2) and the line slope significance level were measured by InStat statistical software.
4. Results
The toxicity results of extractives from four cruciferous plants within 24 hours observation are shown in Tables 1 and 2. As shown in Table 1, the effects of different concentrations of four cruciferous plants namely CD, RS, SN, and CU when applied to brine shrimp at doses ranging from 40 to 400 µg/mL were measured. At the highest concentration of each four-plant extract, which applied to shrimps, the highest mortality was observed at 400 µg/mL concentration of CD extract that was equal to 93%. The rest of the extracts showed mortality levels from 57 to 91% (Table 1). The expected number of the affected shrimp at each concentration of four extractives represents the true relationship between concentration and lethality, further confirmed by drawing their lines and calculation of the X2 values (Table 1). The X2 values calculated for four extractives were less than X2 values of the table that were 3.84 and 5.99 with freedom degree of 1 and 2, respectively (Table 1).
| Test Sample | Con. (µg/ mL) | Log Conc. | Total Noa | No of Dead | % Mortality | Corrected % Mortality | Expected % Mortality | (O - Ea)2 | ∑D | X2b |
|---|---|---|---|---|---|---|---|---|---|---|
| CD | 400 | 2.602 | 30 | 28 | 93.33 | 92.59 | 94.00 | 1.98 | 0.1179 | 3.53 |
| 200 | 2.301 | 30 | 25.00 | 81.76 | 80.46 | 67.00 | 181.17 | |||
| 100 | 2.000 | 30 | 9.00 | 28.86 | 17.94 | 25.00 | 49.84 | |||
| 50 | 1.698 | 30 | 4.00 | 12.20 | 2.48 | 4.00 | 2.29 | |||
| RS | 400 | 2.602 | 30 | 28.00 | 91.10 | 90.82 | 85.00 | 33.87 | 0.1019 | 3.27 |
| 200 | 2.301 | 30 | 19.00 | 61.10 | 59.79 | 57.00 | 7.79 | |||
| 100 | 2.000 | 30 | 9.00 | 28.86 | 26.40 | 25.00 | 1.96 | |||
| 50 | 1.698 | 30 | 5.00 | 15.53 | 12.65 | 6.00 | 44.22 | |||
| CU | 320 | 2.505 | 30 | 26.00 | 86.66 | 85.65 | 89.00 | 11.22 | 0.0379 | 1.14 |
| 160 | 2.204 | 30 | 13.00 | 43.33 | 39.29 | 40.00 | 0.50 | |||
| 80 | 1.993 | 30 | 4.00 | 13.33 | 7.17 | 4.00 | 10.05 | |||
| SN | 160 | 2.204 | 30 | 17.00 | 56.66 | 56.16 | 48.00 | 66.59 | 0.0412 | 1.24 |
| 80 | 1.903 | 30 | 5.00 | 16.66 | 15.75 | 19.00 | 10.56 | |||
| 40 | 1.602 | 30 | 2.00 | 6.66 | 5.72 | 4.00 | 2.96 | |||
| VS | 8 | 0.903 | 30 | 21.00 | 70.00 | 55.83 | 68.00 | 148.11 | 0.1046 | 3.14 |
| 4 | 0.602 | 30 | 13.00 | 43.00 | 35.07 | 44.00 | 79.74 | |||
| 2 | 0.301 | 30 | 9.00 | 30.00 | 20.27 | 23.00 | 7.45 | |||
| 1 | 0 | 30 | 6.00 | 20.00 | 8.88 | 9.00 | 0.01 |
Abbreviations: CD, C. draba; CU, C. uliginosa; RS, R. sylvestris; SN, S. nummularia; VS, vincristine sulfate.
aThree sets of tests used in this bioassay.
bX2 values of Chi - Square distribution table ranging from 3.84 to 5.99 with df = 1 and 2, respectively.
The LC50 values of the four extractives for 24 h assay are shown in Table 2. As shown in this table, the total extracts of CD and SN had a significantly higher mean toxicity against brine shrimp with LC50 values of 147.21 ± 4.13 and 162.97 ± 4.95 µg/mL, respectively. The other two extracts namely the extracts from RS and CU did not show any noticeable cytotoxicity when compared with that of vincristine sulfate and toxicity value of CD extract (Table 2). The equations of the straight lines were obtained from plotting probit values against concentrations and the corresponding squares of the correlation coefficient (R2) for the extractives of CD, SN, RS, and CU were as 0.9752, 0.9630, 0.9940, and 0.9990, respectively.
| Test Samples | LC 50 (μg/mL) | 95% Confidence Limit | Regression Equation | R2 |
|---|---|---|---|---|
| Lower → Upper | ||||
| Ethanolic extract of C. draba | 147.21 ± 4.13 | 113.14 → 191.26 | Y = 3.577 × X - 2.7964 | 0.9752 |
| Ethanolic extract of S. nummularia | 162.97 ± 4.95 | 109.95 → 238.40 | Y = 2.807 × X - 1.232 | 0.9630 |
| Ethanolic extract of R. sylvestris | 169.39 ± 4.29 | 127.91 → 223.97 | Y = 2.780 × X - 1.2269 | 0.9940 |
| Ethanolic extract of C. uliginosa | 176.88 ± 4.80 | 141.00 → 222.44 | Y = 4.950 × X - 6.160 | 0.9990 |
| VS (positive control ) | 5.46 ± 0.76 | 3.38 → 8.97 | Y = 1.993 × X + 3.670 | 0.9980 |
The effects of four cruciferous plant extracts on Artemia salina after a 48 h exposure are shown in Table 3. As shown in this table, the concentrations at which the mortality was above 90% for four extracts ranged from 160 to 320 μg/mL. Among the concentrations used in the entire of extracts, 50 μg/mL concentrations of CD and RS were considered as the least concentration at which the highest mortality was obtained (Table 3). For all extracts, all shrimp larvae used in triplicate tests died, resulting in 100% mortality by using a concentration that was higher than 400 μg/mL. The range of X2 values obtained from the X2 test was from 1.01 to 4.82 when compared with values obtained from X2 table that were from 3.84 to 5.99 with df = 1 and 2, respectively. At 200 μg/mL concentration, the extracts obtained from CD and RS showed the highest mortality while for the extract of CU, the greatest mortality was seen at the concentration of 320 μg/mL. The least mortality of shrimps was related to the extract obtained from SN at 40 μg/mL concentration. There was no death among the shrimps during the test (Table 3).
| Test Sample | Con. (μg/ mL) | Log Conc. | Total Noa | No of Dead | % Mortality | Corrected % Mortality | Expected % Mortality | (O - Ea)2 | ∑d | X2b |
|---|---|---|---|---|---|---|---|---|---|---|
| CU | 320 | 2.505 | 30 | 29 | 96.67 | 94.14 | 94.00 | 0.01 | 0.0559 | 1.67 |
| 160 | 2.204 | 30 | 24.00 | 78.90 | 62.14 | 67.00 | 23.62 | |||
| 80 | 1.903 | 30 | 18.00 | 60.00 | 28.09 | 25.50 | 6.71 | |||
| 40 | 1.602 | 30 | 11.00 | 37.80 | 0 | 4 | 16.00 | |||
| SN | 160 | 2.204 | 30 | 25.00 | 84.47 | 77.55 | 77.00 | 0.30 | 0.0164 | 0.49 |
| 80 | 1.903 | 30 | 14.00 | 45.57 | 21.01 | 25.00 | 15.91 | |||
| 40 | 1.602 | 30 | 10.00 | 33.34 | 3.24 | 2.00 | 1.53 | |||
| RS | 200 | 2.301 | 30 | 28.00 | 94.43 | 79.11 | 80.50 | 1.92 | 0.1606 | 4.82 |
| 100 | 2.00 | 30 | 26.00 | 86.66 | 49.98 | 45.00 | 24.81 | |||
| 50 | 1.698 | 30 | 22.00 | 73.33 | 0 | 13.00 | 169.00 | |||
| CD | 200 | 2.301 | 30 | 28.00 | 94.43 | 83.29 | 80.50 | 7.80 | 0.0339 | 1.01 |
| 100 | 2.00 | 30 | 26.00 | 85.53 | 56.59 | 49.00 | 57.73 | |||
| 50 | 1.698 | 30 | 24.00 | 78.86 | 36.61 | 33.00 | 13.03 | |||
| VS | 2 | 0.301 | 30 | 29.00 | 96.66 | 95.14 | 96.00 | 0.74 | 0.03926 | 1.18 |
| 1 | 0 | 30 | 27.00 | 90.00 | 85.71 | 79.00 | 0.45 | |||
| 0.5 | -0.301 | 30 | 17.00 | 56.66 | 38.00 | 43.00 | 0.25 |
Abbreviations: CD, C. draba; CU, C. uliginosa; RS, R. sylvestris; SN, S. nummularia; VS, vincristine sulfate.
aThree sets of tests used in this bioassay.
bX2 values of Chi - Square distribution table ranging from 3.84 to 5.99 with df = 1 and 2, respectively.
The LC 50 values of the four extracts are shown in Table 4. As shown in this table, the least amount of LC50 (the greatest toxicity) was related to CD extract with LC50 equal to 78.17 μg/mL, followed by RS extract (105.37 μg/mL), SN extract (110.49 μg/mL), and CU extract (120.97 μg/mL) in increasing order of LC50 value. The LC50 value calculated for VS, which was used as a positive control, was 1.02 μg/mL (Table 4). There were no wide confidence intervals for calculated LC50 values of each of the plant extracts (Table 4). The correlation coefficients (R2) obtained from drawing the straight line associated with dose and response curve (mortality % in brine shrimp larvae) for the extractives of CD, RS, SN, and CU used in 48 h LC50 tests were as 0.9820, 0.9986, 0.9810, and 0.9970, respectively, that all were close to the unity.
| Test Samples | LC 50 ( μg/mL) | 95 % Confidence Limit | Regression Equation | R2 |
|---|---|---|---|---|
| Lower → Upper | ||||
| Ethanolic extract of C. draba | 78.17 ± 3.10 | 57.33 → 106.57 | Y = 2.12 × X + 0.9779 | 0.9820 |
| Ethanolic extract of R. sylvestris | 105.37 ± 2.86 | 82.03 → 135.34 | Y = 3.2721 × X - 1.668 | 0.9986 |
| Ethanolic extract of S. nummalaria | 110.49 ± 3.28 | 81.32 → 150.12 | Y = 4.518 × X - 4.224 | 0.9810 |
| Ethanolic extract of C. uliginosa | 120.97 ± 2.73 | 96.03 → 152.37 | Y = 3.591 × X - 2.457 | 0.9970 |
| VS (positive control ) | 1.02 ± 0.18 | 0.78 → 1.34 | Y = 1.545 × X + 6.437 | 0.9935 |
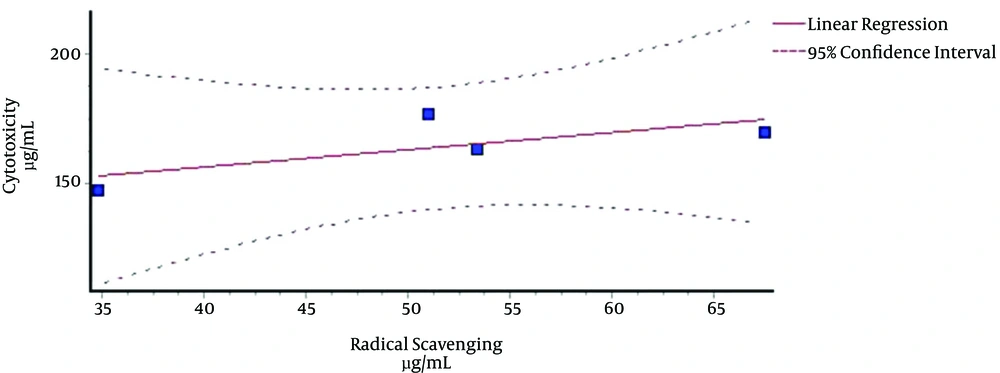
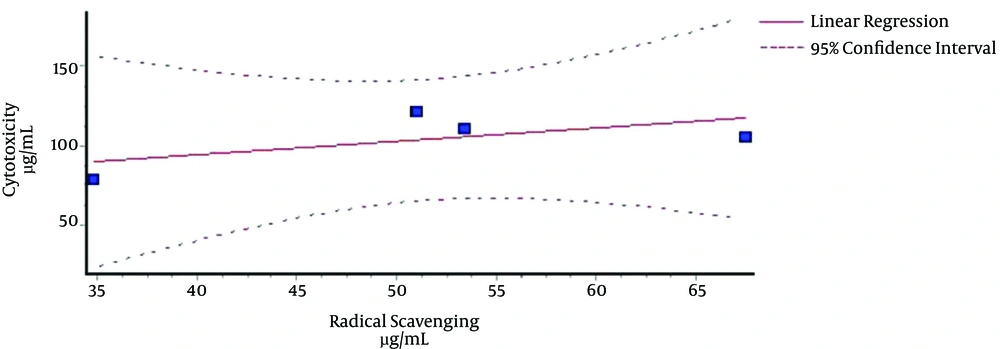
The analyses of correlations between the two sets of lethality bioassays at 24 and 48 h against shrimps and IC50 values of the four extracts for their scavenging activities revealed that there were no significant correlations between them (Figures 1 and 2). The correlation coefficients in Figures 1 and 2 are R2 = 0.4981 and R2 = 0.388, respectively.
5. Discussion
One of the noticeable methods that can be used for toxicity assessment in medicinal plants is brine shrimp bioassay. It has been used for a preliminary detection of toxicities related to toxic fungi, plants, heavy metals, pesticides, dental materials, and chemicals for cancer therapy (22, 23). This approach also has been accepted as an outstanding simple method by which, the biological activity of the medicinal plants could be determined (24). In fact, by using this approach, the need for applying animals in toxicological experiments will be reduced (25). It is a robust method in which the median lethal concentration (LC50) is used for determination of toxicity (21).
Our results obtained from 24 h and 48 h toxicities against brine shrimp (Tables 1 - 4) showed that CD extract was the most toxic among crude extracts that were screened. However, it seems that the manner of its toxicity is time - dependent; the more time passes, the greater toxicity will be against shrimp and accordingly, the greater effects will be seen. The highest lethal concentration of extracts against shrimp was related to the extract from CU. From these data, it is evident that CD and CU extracts have the most and the least activities in brine shrimp tests among the four cruciferous plant extracts screened for their cytotoxicity. CD is a plant from Cruciferae family that mostly grows in some districts of Iran such as Karaj and north area of Alborz (26). The active ingredient of this plant undergoes enzymatic breakdown and finally converted into very bioactive compounds namely isothiocyanates (27, 28), which are believed to cope with some cancers such as prostate and breast cancer (10, 13, 29). One of the reasons that the toxicity increased in CD plant extract so much so that it was more toxic compared to the other three extracts may be due to the amount of sulfur - containing compounds present in CD plant. Since the GLS amounts more than 0.4% are very toxic to livestock, this issue may have caused CD to be more toxic than the other extractives (18, 19). In addition, here it should be said that the type of glucosinolates and metabolic by - products exclusively present in this plant, namely sulforaphane compounds, is different from those in other plants that may consequently cause an increased toxicity when compared to others. In addition, the mentioned compounds may have a role in protective properties against the treatment of the above - mentioned cancers. Plant extracts with LC50 lower than 1 mg/mL have a potential for toxicity and are considered active plants (21, 30, 31). Therefore, it seems that CD plant with LC50 far below 1 mg/mL is biologically active and may show its toxic expressions in animal cells.
The correlation analysis between free radical scavenging activities of the whole four extracts and their cytotoxicities (Figures 1 and 2) showed that there were not any significant correlation coefficients. Although there was no positive relationship between cytotoxicities of the extracts and free radical scavenging, our previous studies on antioxidant effects of these four cruciferous plants showed that they are able to somewhat remove radicals in test tubes and show noticeable antioxidant properties (data not shown) (4, 14).
Most anticancer drugs used for the treatment of malignant tumors have side effects and can exert overt toxicities in healthy cells. The anticancer drug that was used as a positive control was vincristine. VX has been successfully used for the treatment of some cancers like acute leukemia in children and it is useful in the treatment of very rapidly proliferative neoplasm (32). It is known as mitotic poison and a series of side effects and delayed toxicities such as peripheral neuritis, muscle weakness, and bone marrow depression have been attributed to this drug (33). In our studies, all LC50 values of extractives were higher than that of VX. However, this difference was fewer regarding the extract of the CD. At present, the worldwide concentration on anticancer drugs is on plant - derived substances that have biologically active components as well as antioxidant effects (20). Our previous in - vitro studies on CD showed that this plant has a potential to remove successfully free radicals when compared with Vit - C (4). If our findings in terms of CD are taken together, it seems that CD through its isothiocyanate formation pathways, its polyphenol contents, and its GLS entities may inhibit proliferating cells in some cancers. Owing to its antioxidant effects, its presumable side effects may be less than side effects of some regular plant anticancer drugs like VX. However, more studies must be done in this respect to show whether this presumption is true or not.
5.1. Conclusions
From the data of this study, it can be concluded that CD extract has potential in - vitro cytotoxic activity. Since toxicity of natural products can be relevant to their pharmacological effects, the extract of C. draba plant from Iran has the highest bioactivity among other extracts screened for their cytotoxic effects. However, further complementary studies must be carried out on its anticancer properties in cell lines.