1. Background
Hematologic malignancies and solid tumors are responsible for at least 27% of deaths all over the world (1-3). Acute myeloid leukemia (AML) is the most common acute leukemia in adults (4, 5). Problems and complications can be caused by malignancy itself or the side effects of therapies e.g. radiotherapy, chemotherapy, hematopoietic stem cell transplantation or a combination of these treatment modalities (6, 7).
Cytotoxic anti-neoplastic agents (chemotherapeutic drugs) are used at different stages of AML treatment. Oral mucositis is one of the oral cavity complications caused by chemotherapy. It is a common side effect of chemotherapy, which occurs in 30% to 40% of patients (8) and is a dose-limiting toxicity (9). This complication makes patients unable to eat, swallow or speak (10). Early symptoms of oral mucositis are seen three to five days after initiation of chemotherapy regimen with a peak on days 7 to 14 after the start of regimen and could expand to three weeks (11).
Achillea millefolium, known as yarrow, is a member of the Asteraceae family, which has been used in traditional medicine in many countries, especially in Europe and Asia (12). Yarrow has been used in treating hepatobiliary complaints, relief of inflammation, and spasm of gastrointestinal tract. In addition, Yarrow is used as an appetizer, wound healer, and an antiulcer and anti-inflammatory agent (13). In addition, the extract of Achillea millefolium has anticancer effects on different types of malignancies, including leukemia, cervical and breast epithelial adenocarcinoma, skin epidermoid carcinoma, hepatoma, and lung tumor cells (13).
The main components of Achillea millefolium include flavonoids, phenolic acids, alkaloids, terpenes (cineole, borneol, pinene, camphor and azulen), tannins, cis-carveol, achillin, and leucosis. Flavonoids and phenol carbonic acids constitute the most important groups of pharmacologically active substances of Yarrow (14). Flavonoids are also effective on gastric mucosal lesions and in addition to their antimicrobial and antioxidant effects, they are gastro-protective (15). Flavonoids also have anti-ulcer effects on gastric lesions (16).
In a randomized clinical trial, Miranzadeh et al. studied the effect of Achillea millefolium distillate solution on the improvement of chemotherapy-induced oral mucositis. The results showed that Achillea millefolium distillate is more effective than the routine solution in recovery of oral mucositis (17). Achillea millefolium mouthwash is used in patients with AML.
The most responsible chemotherapeutic agents, which cause mucositis are methotrexate, fluorouracil, cytarabine, doxorubicin, etoposide, melphalan, and bleomycin. Patients with AML receive at least two of these oral toxic agents (18).
There are no acceptable agents with remarkable effect on prevention and treatment of oral mucositis. Due to Achillea millefolium’s anti-inflammatory effect, it is a suitable choice for formulating a mouthwash and evaluating its effect on oral mucositis.
2. Objectives
This study proposes to examine the effectiveness of Achillea millefolium mouthwash on oral mucositis, induced by chemotherapy in patients with AML.
3. Methods
3.1. Extraction
Aerial parts of Achillea millefolium at the flowering stage were collected, dried at room temperature, and subsequently milled to a fine powder. Thereafter, 1000 g of the dried plant material was macerated in ethanol: Water (70:30). The extract was filtrated through Whatman No. 1 filter paper. The alcohol of the resultant extract was removed by rotary evaporator at 50°C. Sodium benzoate (0.05%) was then added as a preservative.
3.2. Formulation
The experiments showed that the suitable co-solvent for Yarrow’s extract was Brij 35. Brij 35 (5%) was suitable for preparation of Yarrow’s extract water solution. Then salicylic acid (0.5%) is added as an anti-microbial preservative. In addition, salicylic acid increases Yarrow’s extract penetration into oral cells (19). Menthol (0.5%) was added after dissolving in a small amount of ethanol. At the end, mannitol (10%) subjoined as a flavor and coolant. Finally, Yarrow’s extract water-base mouthwash (3%) was prepared.
3.3. Patient Enrollment
In this randomized clinical trial, a total of 30 patients with AML, who had undergone induction or consolidation chemotherapy at Shahid Ghazi Hospital in Tabriz, Iran, enrolled in the study. Patients were randomized by computerized random numbers.
Induction or consolidation chemotherapy patients with 7 + 3 or 5 + 2 regimen were assigned to intervention (n = 15) and control (n = 15) groups. The 7 + 3 regimen combines a seven-day continuous intravenous infusion of cytarabine (100 or 200 mg/m2 per day) with a short infusion or bolus of an anthracyclines (daunorubicin 30 to 90 mg/m2 per day or idarubicin 12 - 13 mg/m2 per day), administered on days one to three. The 5 + 2 regimen consist of a five day continuous intravenous infusion of cytarabine 100 mg/m2 per day with a bolus of an mitoxantrone 12 mg/m2 per day or idarubicin 13 mg/m2 per day given on days one and two. Patients younger than 14 and/or known to have allergy to Achillea millefolium or chlorhexidine mouthwashes were excluded from the study.
3.4. Study Protocol
Routine therapy for prophylaxis and treatment of oral mucositis in Ghazi Hospital is using the chlorhexidine mouthwash, and therefore, both groups (intervention and control) were instructed to use the chlorhexidine mouthwash.
In both groups, patients were instructed to rinse with 10 mL of chlorhexidine mouthwash two times per day for 20 days. In the intervention group, patients were instructed to rinse with additional 10 mL of Achillea millefolium mouthwash twice a day from the first day of chemotherapy. Patients oral mucositis pain was assessed with pain questioner in order to categorize the pain to mild, medium and severe. Patients with severe pain received painkillers, such as different kinds of NSAIDs and opioid. Examination and scoring of oral mucositis were performed on the first, 5th, 10th, 15th, and 20th days of chemotherapy. For scaling oral mucositis, the World Health Organization (WHO) mucositis grading scale was used (Table 1).
| Grade | Description |
|---|---|
| Mild | |
| 0 (none) | None |
| I (mild) | Oral soreness, erythema |
| II (moderate) | Oral erythema, ulcers, solid diet tolerated |
| Severe | |
| III (severe) | Oral ulcers, liquid diet only |
| IV (life-threatening) | Oral alimentation impossible |
3.5. Statistical Analysis
Normality of quantitative data was assessed with the Kolmogorov-Smirnov (K-S) test. Independent t-test was used to compare quantitative variables. Ordinal data were analyzed by the Mann-Whitney U test. Chi-square test (or Fisher’s exact test if needed) was used to compare qualitative results. P values less than 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS statistics for Windows, version 16.0.
3.6. Ethics
The study protocol was approved by the Research Ethics Committee of Tabriz University of Medical Sciences. All patients were informed about the trial and gave their written informed consent before the study initiation. This clinical trial was submitted in the Iranian Registry of Clinical Trials (IRCT) with registration number of IRCT201309215704N2.
4. Results
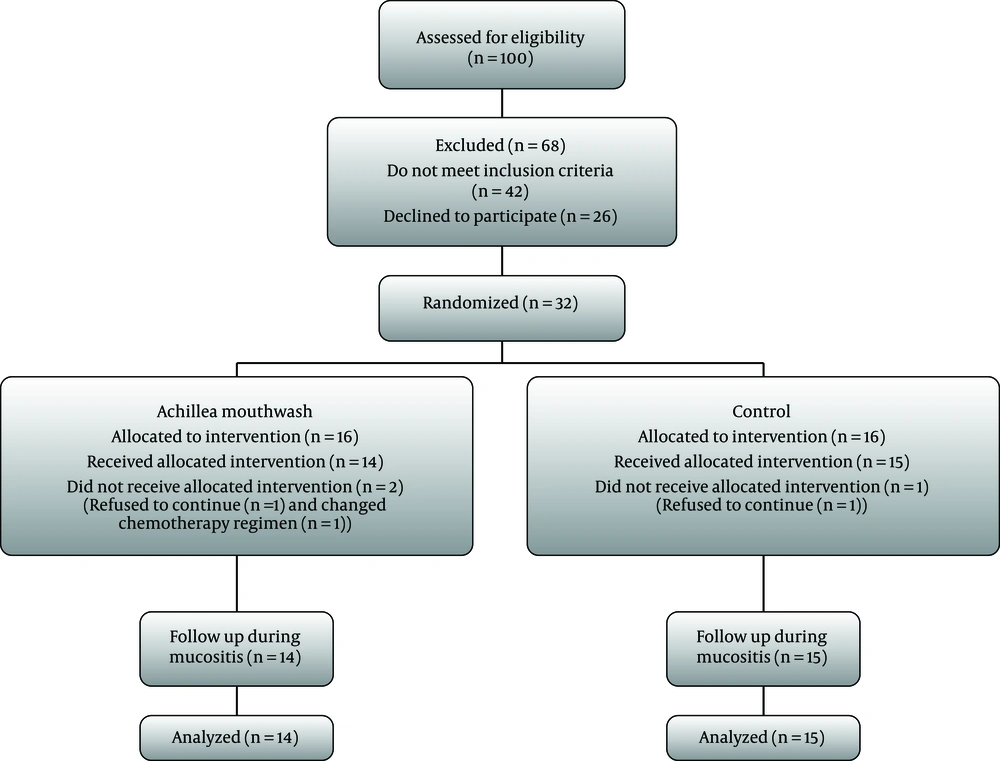
From 100 patients screened to be included in this study, 42 did not meet the inclusion criteria, and 26 refused to participate in the trial. Finally, 32 patients were randomized to two groups. There were three dropouts during the study, including two in intervention group and one in the control group. Therefore, 29 patients, 14 in the intervention and 15 in the control group, completed the period of the study (Figure 1). No side effects were reported by patients other than bad taste of Achillea millefolium mouthwash. All patients tolerated the mouthwash well and the amount of consumption was assessed daily by the volume of reduction of whole bottle and verbal interview. Patients, who had developed oral mucositis during chemotherapy were 11 in the intervention and 13 in the control group. There were no remarkable differences in baseline characteristics between intervention and control groups (Table 2). The mucositis occurrences during all treatments were not statistically different between groups (P = 0.58).
| Parameters | Intervention, n = 14 (%) | Control, n = 15 (%) | P Valuea |
|---|---|---|---|
| Gender | |||
| Male | 5 (35.7) | 7 (46.66) | 0.550 |
| Female | 9 (64.3) | 8 (53.33) | 0.550 |
| History of other blood illness | 0.292 | ||
| Yes | 1 (7.14) | 0 (0) | |
| No | 13 (92.85) | 15 (100) | |
| History of oral illnesses | 0.837 | ||
| Yes | 8 (57.14) | 8 (53.33) | |
| No | 6 (42.85) | 7 (46.66) | |
| Dentist visit in last year | 0.176 | ||
| Yes | 4 (28.57) | 8 (53.33) | |
| No | 10 (71.42) | 7 (46.66) | |
| Oral hygiene used | 0.588 | ||
| Toothbrush | 7 (50) | 6 (40) | |
| Mouthwash | 4 (28.57) | 7 (46.66) | |
| Nothing | 3 (21.42) | 2 (13.33) | |
| History of oral ulceration | 0.159 | ||
| Yes | 9 (64.28) | 13 (86.66) | |
| No | 5 (35.71) | 2 (13.33) | |
| Chemotherapy regimen | 0.941 | ||
| 7 + 3 | 12 (85.71) | 13 (86.66) | |
| 5 + 2 | 2 (14.28) | 2 (13.33) | |
| Ageb | 44.71 ± 16.6 | 41.86 ± 12.50 | 0.605 |
aP values are generated by chi-squared test.
bData were expressed as mean ± SD.
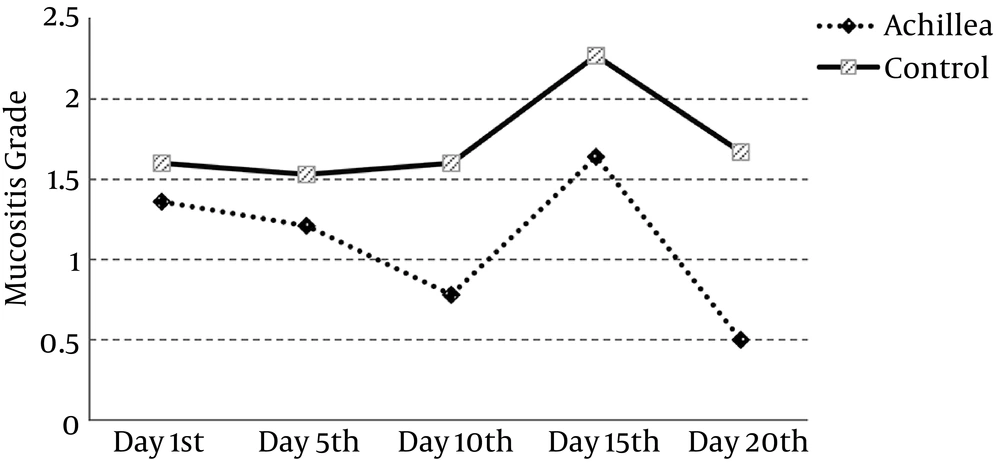
Although the results show that mucositis grade improves on all days during treatment in the intervention group, differences occurred on the 10th and 20th day in comparison with the control group (P value 0.021 and 0.002, respectively) (Table 3). That being said, pattern of oral mucositis during treatment has been shown in Figure 2.
| Mucositis Grade | Intervention, n = 14 | Control, n = 15 | P Valueb |
|---|---|---|---|
| Day 1st | 1.36 ± 1.34 | 1.60 ± 1.64 | 0.681 |
| Day 5th | 1.21 ± 1.05 | 1.53 ± 1.41 | 0.527 |
| Day 10th | 0.78 ± 0.80 | 1.60 ± 0.91 | 0.021 |
| Day 15th | 1.64 ± 1.00 | 2.27 ± 1.22 | 0.124 |
| Day 20th | 0.50 ± 0.52 | 1.67 ± 1.11 | 0.002 |
aValues are expressed as mean ± SD.
b P values are generated by Mann-Whitney U test.
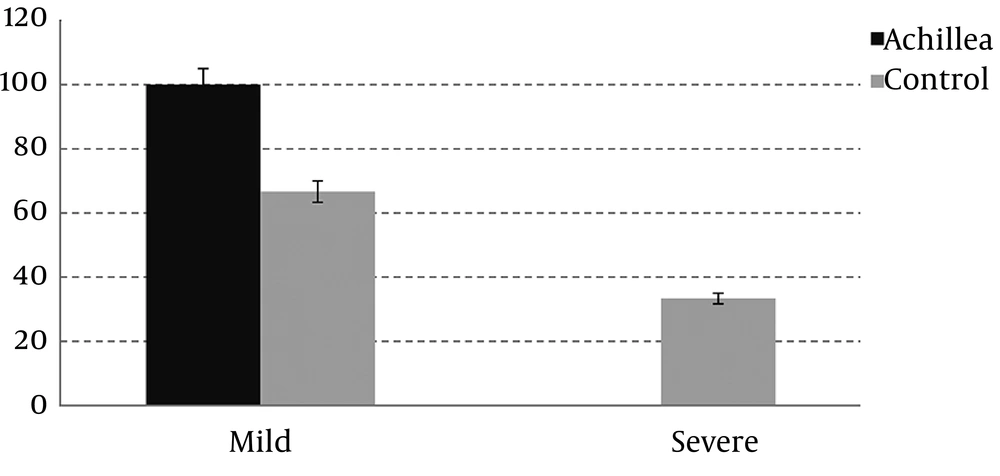
Mucositis is divided to two severe (grade 3 and 4) and mild forms (grade 0, 1 and 2). Data show that the incidence of severe mucositis is significantly higher in the control group (P = 0.018) (Figure 3).
The occurrence of pain in all duration of treatment is not statistically different between groups (P value 0.674, respectively). However, number of patients, who had pain in the intervention group was lower than the control group and the difference was significant on the 20th day of study (P = 0.002).
According to pain pattern, although amount of painkiller requested by patients were continuously lower in the intervention group, this difference was only significant on the 20th day (P value 0.001).
5. Discussion
Oral mucositis has been characterized by damages to the epithelium of oral cavity, pharynx, and gastrointestinal tract from chemotherapy or radiotherapy for malignancies (20). There is a need to identify better therapies for chemo-therapy-induced oral mucositis. Chlorhexidine mouthwashes are administrated in order to apply across the surface of the oral cavity without specific effects on lesions, except the antimicrobial effect (21).
Negrin et al. showed that there was not any remarkable difference between the chlorhexidine and placebo groups in the severity of oral mucositis (18). In addition, their study showed that there was no beneficial advantage in using chlorhexidine mouthwash for mucositis prevention in patients with bone marrow transplantation (22).
Natural products are used in traditional medicine to relieve oral ulcer, however, this is not well documented. Various plant-based products have been identified to treat mucositis (23). Previous studies showed that flavonoids had anti-ulcer activity and also various plants, containing saponins and tannins, possess antiulcer activity (24). It is likely that presence of flavonoids, tannins, and other bioactive components in Yarrow may have played an important role in prevention and healing of oral mucositis (25).
In a study by Rashidi et al., Yarrow distillate showed antibacterial and healing effects on treatment of rats’ gastric ulcer (26). Miranzadeh et al. studied the effect of Achillea millefolium distillate solution in the treatment of chemotherapy-induced oral mucositis and stomatitis (15). They compared the effect of routine solution (1400 mg of Lidocaine, 224 mg of dexamethasone, 35,000 mg of Sucralfate per liter to a Diphenhydramine solution) and distilled Achillea millefolium with routine solution. The control group received the routine mouthwash while patients in the experimental group received a mixture of the routine mouthwash and Achillea millefolium distillate (50/50). The results showed that Achillea millefolium distillate healed oral mucositis more than the routine solution (17).
These findings are in line with previous reports indicating beneficiary effects of Achillea millefolium in prevention and treatment of chemotherapy-induced oral mucositis. In the current study, Achillea millefolium extract mouthwash was formulated separately from routine solution and patients were instructed to use it separately, however, in the previous study (15) distilled Achillea millefolium was mixed with routine solution. Furthermore, the current study formulated a water-base mouthwash from Achillea millefolium extract while Miranzadeh et al. used distillate Achillea millefolium.
In this randomized clinical trial, the researchers evaluated the prophylactic and curative (healing) effects of Achillea millefolium extracts with chlorhexidine mouthwash in comparison with chlorhexidine mouthwash alone against chemotherapy-induced oral mucositis.
Oral mucositis incidence was the same in both groups. This shows that Achillea millefolium mouthwash does not have any prophylactic effect on occurrence of oral mucositis. Although the incidence of mucositis was not changed significantly, mucositis grade was improved in the intervention group on the 10th and 20th day.
Following the pattern of pain occurrence, amount of painkillers consumption during treatment are lower in the intervention group than in the control group. From the 10th day, painkiller consumption trend changes and shows significant difference on the 20th day (decreasing trend in intervention and increasing trend in the control group). Consequently, patterns of number of patients with pain and painkiller consumption in both groups followed the pattern of mucositis grade.
Although Achillea millefolium mouthwash does not show any prophylactic effect on occurrence of mucositis, severity of oral mucositis is significantly lower in the experimental group. Patients using Achillea millefolium mouthwash have shown faster recovery and lower oral mucositis grade during treatment.
5.1. Conclusions
In conclusion, Achillea millefolium mouthwash decreases the severity of oral mucositis. Subsequently, patients recover faster and mucositis-related complications are reduced. Therefore, Achillea millefolium can be administrated in patients, who are receiving chemotherapy for AML treatment.
Limitations of this study were the low number of patients, lack of different percentages of Achillea millefolium mouthwash solution, and the same ingredient except Achillea millefolium as a control group.
5.2. Study Limitations
This study was not blind. Double-blind and placebo-controlled studies are suggested in the future.