1. Introduction
Malaria continues to be an important febrile infectious disease in some areas of the world (1, 2). Out of 5 Plasmodium species that infect humans, Plasmodium vivax is the most prevalent in the malaria endemic regions of Iran (3). The number of Plasmodium vivax sporozoites that get to the liver remain latent (hypnozoite). After several months or years of inappropriate treatment period, hypnozoites can reactivate and again initiate tissue and blood schizogony that leads to the clinical manifestation of malaria (4-6).
It is not usually possible to make a diagnosis of a relapse from a new infection in malaria endemic areas, where new malaria cases still occur due to transmission of Plasmodium by mosquito biting (7-9). Meanwhile, malaria patients who leave the malaria endemic areas after the treatment period are appropriate evidence for the exhibition of a relapse.
Emergent relapses in areas such as Iran, which have planned to eliminate malaria, could be considered as an obstacle for accessing the elimination, due to the fact that malaria parasites are again introduced to the mosquito’s vector via relapsed malaria patients and vectors that continue to transmit parasites to humans. In this case report, we describe the appearance of clinical P. vivax malaria in a patient out of the malaria endemic area. The patient had a history of clinical malaria and treatment with chloroquine and primaquine in Chabahar, which is the malaria endemic area of Iran.
2. Case Presentation
A 49-year-old male was referred to a private clinical center in the capital city of Iran, Tehran, in October 2016, due to fever, chills, nausea, appetite, headache, and weakness. The patient had a history of clinical vivax malaria 1-year prior in Chabahar, in the province of Sistan and Baluchistan. The province of Sistan and Baluchistan is located southeast of Iran and bordering Pakistan and Afghanistan. This province is the main malaria endemic area of Iran. However, Tehran is a region free from malaria and there has been no malaria transmission in at least the past 4 decades (10). The patient didn’t travel to any malaria endemic areas and he had no risk of exposure to the malaria infection during the previous year.
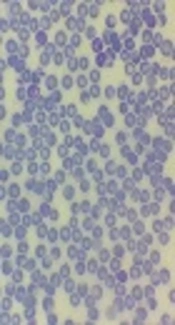
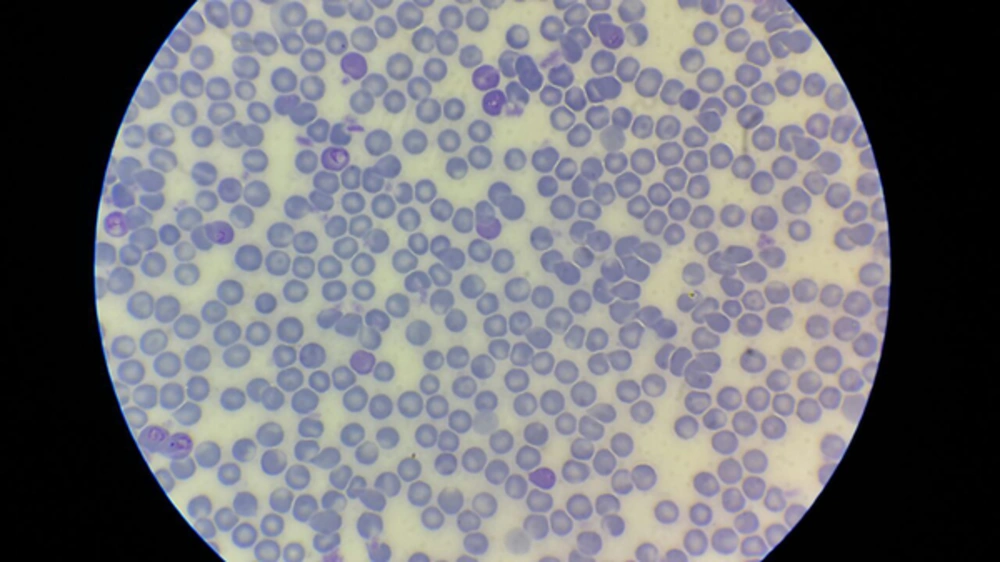
The introduction of laboratory examinations indicated white blood cells of 9,300/µL with neutrophil 88.5%. The haemoglobin was 11.7 g/dL. The patient’s platelet level was 156,000/µL. Microcytic hypochromic RBC was reported. Trophozoite and schizont forms of Plasmodium vivax were observed in Giemsa peripheral blood smear under a light microscope (Figure 1). Other laboratory test findings were in the normal ranges.
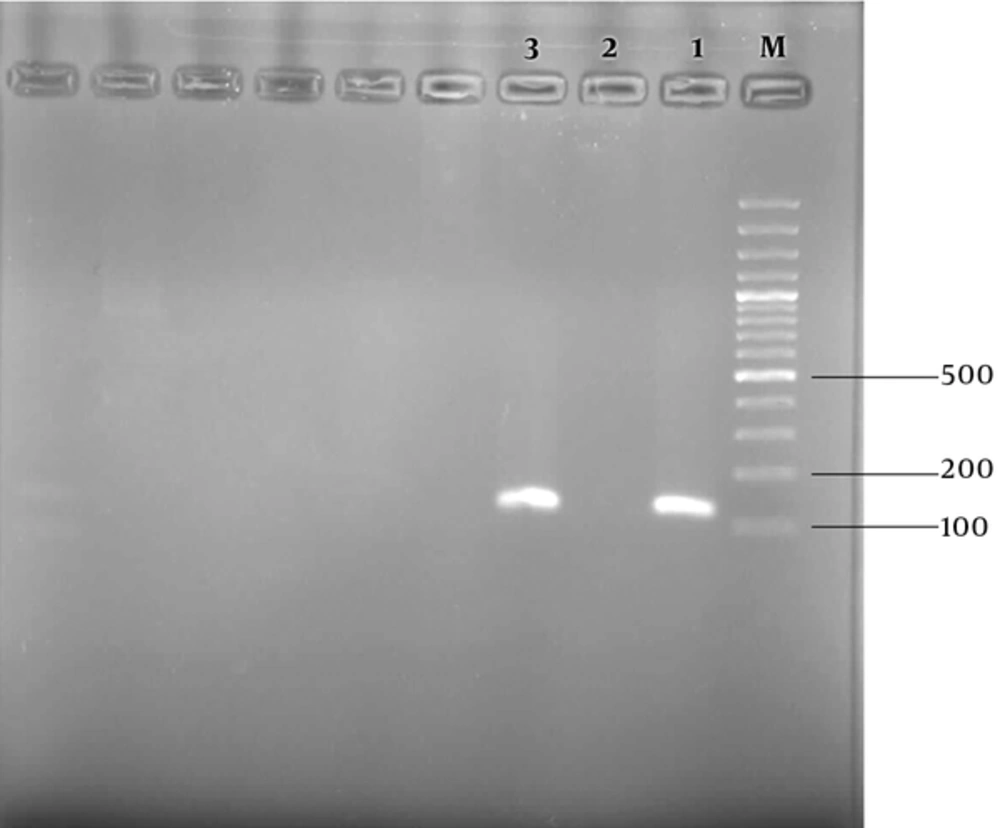
The nested PCR using the Plasmodium 18 subunit ribosomal RNA gene was applied for confirming the vivax malaria infection. The DNA of the patient’s blood was extracted using a Qiagen Kit. The nested PCR product was approximately 120 bp in agarose gel electrophoresis. Therefore, the existence of P. vivax parasites in the blood was confirmed by nested PCR data (Figure 2). Primers and a detailed nested PCR amplifying method have been described previously (11). The patient was treated for 3 days with a regimen of chloroquine as anti-malaria blood schizonticide and primaquine for the resolution of the latent forms in the liver, according to the protocol of the Iranian Ministry of Health and Medical Education. His fever and symptoms were completely resolved at the day-1 follow-up. The thick and thin peripheral blood smears on days 7, 14, and 28 were negative.
3. Discussion
During the initial infection, the patient had been treated with chloroquine as a blood schizonticide and primaquine as a radical cure for the resolution of hypnozoites in the liver, in Chabahar, 1-year prior to the occurrence of the relapse. Primaquine, 8-aminoquinoline,, has remained the only drug to resolve the latent phase of P. vivax in the liver (2, 9, 12). Primaquine can lead to deadly hemolysis in patients with glucose 6 phosphate dehydrogenase (G6PD) deficiency (2). According to World Health Organisation (WHO) protocols for preventing severe hemolysis, in countries with high rate of G6PD deficiency individuals, primaquine is administrated at 45 mg per week for 8 weeks instead of the usual regimen of 14 days afterwards by the Iranian Ministry of Health and Medical Education (13). It seems that the surveillance of this regimen encountered some difficulties due to the longitude of the regimen duration, and the patients may not carefully obey the primaquine doses in the 8-week regimen, specifically when they feel better and their clinical manifestations disappear. There are some reports of P. vivax relapses in the world with using an inadequate dosage of primaquine (7, 9). It has been noted that there are geographical variations in the emergence and periodicity of P. vivax relapses from 3 weeks to 3 years following the initial infection (2, 14). Despite tropical strains of P. vivax that are described with short latency, the strains in temperate and subtropical areas have a tendency to illustrate a long latency (2, 7).
In this patient, the relapse of P. vivax occurred after about 12 months following the primary malaria infection. Sistan and Baluchistan has a temperate to subtropical climate. In addition, it seems that the relapse happened after a longer time in comparison with the tropical strains, such as Chession. The tendency of P. vivax to persistently form liver stage malaria parasites causes more difficulties in the control and elimination of vivax malaria than Plasmodium falciparum in endemic areas including southeast of Iran.
3.1. Conclusions
The report underlines that relapses should be considered in febrile individuals returning from malaria endemic areas of the country to malaria free areas, as well as, highlights the occurrence of relapse in the patient with history using primaquine for a radical cure of liver hypnozoites. Therefore, more surveillance and monitoring should be applied for correct consuming primaquine. Finally, the emergence of P. vivax relapses in the endemic region of Iran could hinder the elimination malaria program.