1. Background
Perovskia Karel. is a small genus belongs to Lamiaceae family, represented in Flora Iranica by only three species. They are native to central Asia in an area that includes Afghanistan, Iran, Pakistan, and Tibet (1, 2). Perovskia abrotanoides Karel is an aromatic herb, and was used in Iranian folk medicine as an analgesic in rheumatic pains (3-5). P. abrotanoides is used for the treatment of cutaneous leishmaniasis (5, 6). Also, this species is suitable for forming a decorative hedge of moderate height (5). Many species of Lamiaceae produce volatile oils, which are secreted by their glandular trichomes on aerial vegetative organs and some reproductive organs (7).
There are a lot of investigations about the influence of environment on glandular trichomes and composition of essential oil in other species. Furthermore, there are some studies about different methods of obtaining volatile oil. For instance, sun drying was an efficient way to obtain volatile oil from Ocimun basilicum compared with other methods. Volatile oil was found to accumulate mostly in the flowers and little in the stem (8).
In this study, the essential oils of the aerial part of Perovskia atriplicifolia were isolated by steam distillation at three stages of growth (before flowering, at the beginning of flowering, and flowering was completed), and declared that these three factors (the part of plant, the method of distillation, and the stage of growth) have high effects on essential oil compounds (9). Moreover, geographic distances influence the chemical variability of Valeriana jatamansi (10). The involvement of abiotic factors like temperature and drought are very influential in the variety of chemical compositions in Origanum vulgare, but they do not affect the morphology of trichomes (11).
The chemical composition of essential oil obtained by hydrodistillation is influenced by the condition of plant growth. The plant morphology and essential oil composition characterized, and the effects of genotype and environment were assessed. Because of the environmental conditions, the biotype belonged to two different subspecies: Origanum vulgar ssp. Virens and Origanum vulgare ssp. Viridulum (12). Furthermore, the study about the chemical composition of P. abrotanoides conducted in Pakistan identified 26 compounds. Major constituents were 1,8-cineol and δ-3-carene, which constitute 50% of the oil.
These studies showed that the environment does not have significant effects on major constituents although the environment effects are so clear in other elements such as caren-3-β, α-elemene, etc. (13). The thermal stability of leaf essential oil from various Cinnamomum osmophloeum spp. was investigated, and the results indicated that essential oil from this species was affected by temperature (14). In addition, temperature influences the resistance level of glandular hair in Medicago sativa (15). Glandular secreting trichomes of cultivated tomato (Solanum lycopersicum) produce a wide array of volatile and nonvolatile specialized metabolites. Many of these compounds interact with other species in the natural environments (16), so they are affected regarding phenotypes, including alteration in the morphology, density and chemical composition of the glandular trichomes. Generally, in contrast to our understanding of nonglandular trichomes, much less is known about the development of the ecological function of the glandular trichomes, many of them are multicellular. Systematic characterization promises to provide new insight into biochemical pathways and ecological function of tomato leaf volatile oil (17).
2. Objectives
The purpose of this study was to investigate the morphology and distribution of glandular trichomes on P. abrotanoides leaves, and the composition of the volatile oil produced by the plant in reproductive stage, collected from different environmental conditions in Iran.
3. Materials and Methods
3.1. Plant Materials
The flower parts of Perovskia abrotanoides Karel. (at their flowering stage) were collected from Sistan and Baluchistan Province by Ms. Salimi and from Khorasan Province by Dr. Joharchi during May 2013. The plant was identified by botanist; Dr. Lili Ghaem Maghami at the Botany Department of Isfahan University. Moreover, the plant sheets were kept in the herbarium by 16278 voucher specimen number in Science Faculty of Isfahan University. For anatomical analysis, the vegetal material (leaves) was fixed and conserved in fixator (alcohol 70%-glycerin-water 1:1:1). Cross sections were colored with methyl blue-Carmine alum (18). The photos were taken with an Olympus DP12 photo camera.
3.2. GC-MS Analysis
The air-dried flower of the plant was powdered. The essential oil of the powdered flower was obtained using hydrodistillation. Then, essential oil was injected to GC-Mass apparatus (Agilent 6890) equipped with a HP-5MS fused silica column (30 m × 0.25 mm: film thickness 0.25 µm) and interfaced with an Agilent 5975 mass selective detector. The oven temperature was programmed from 60°C to 280°C at a rate of 4°C/min. Helium was used as carrier gas at a flow rate of 2 mL/min. Other conditions of the instrument were as follows: ionization voltage, 70 eV; injector temperature, 280°C; and ion source temperature, 200°C. Identification of the oil components were based on GC retention indices relative to n-alkenes and computer matching with the WILEY 275.L library, as well as by comparison of the fragmentation patterns (of the mass spectra) with those reported in the literature (19, 20).
4. Results
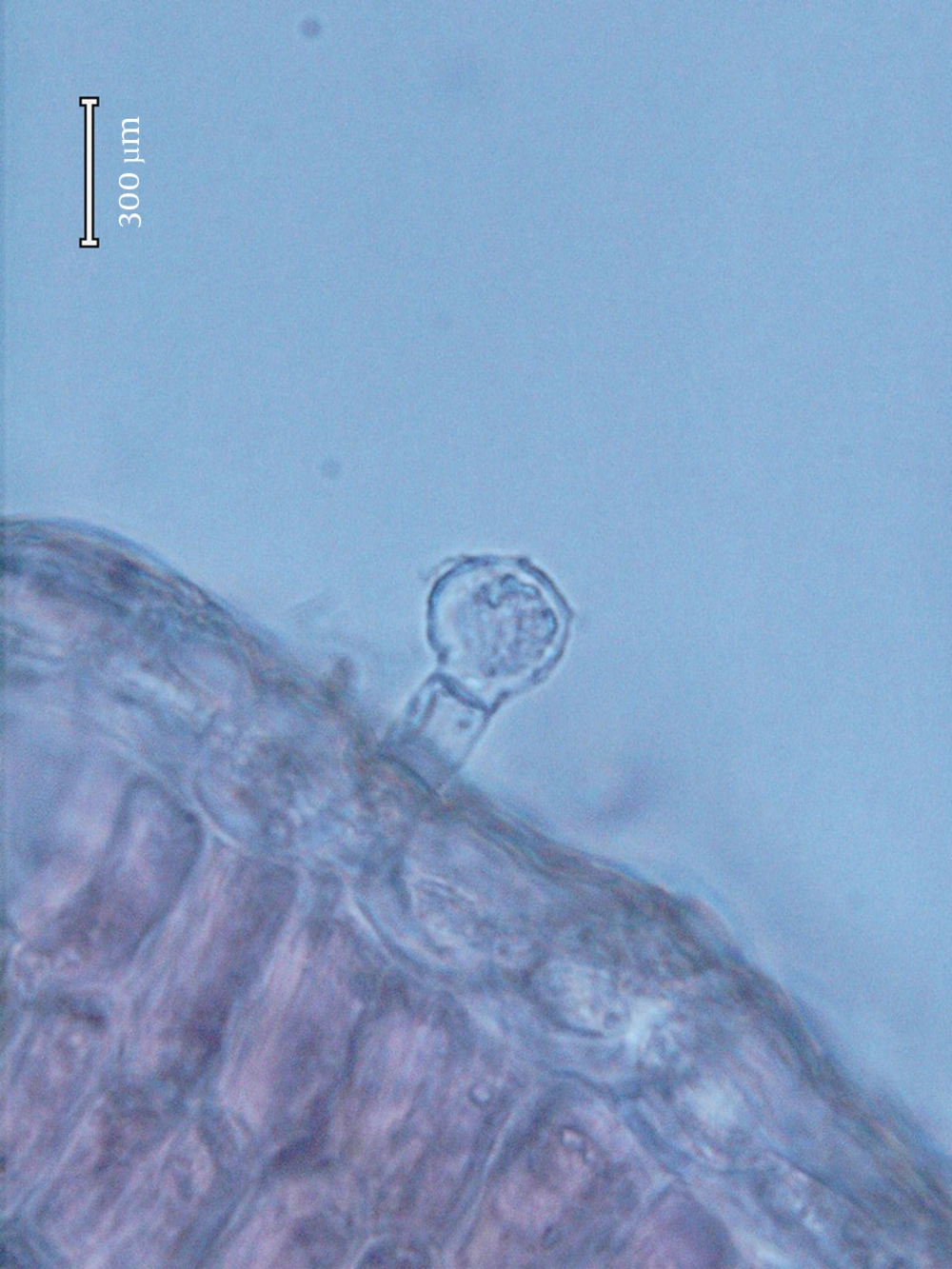
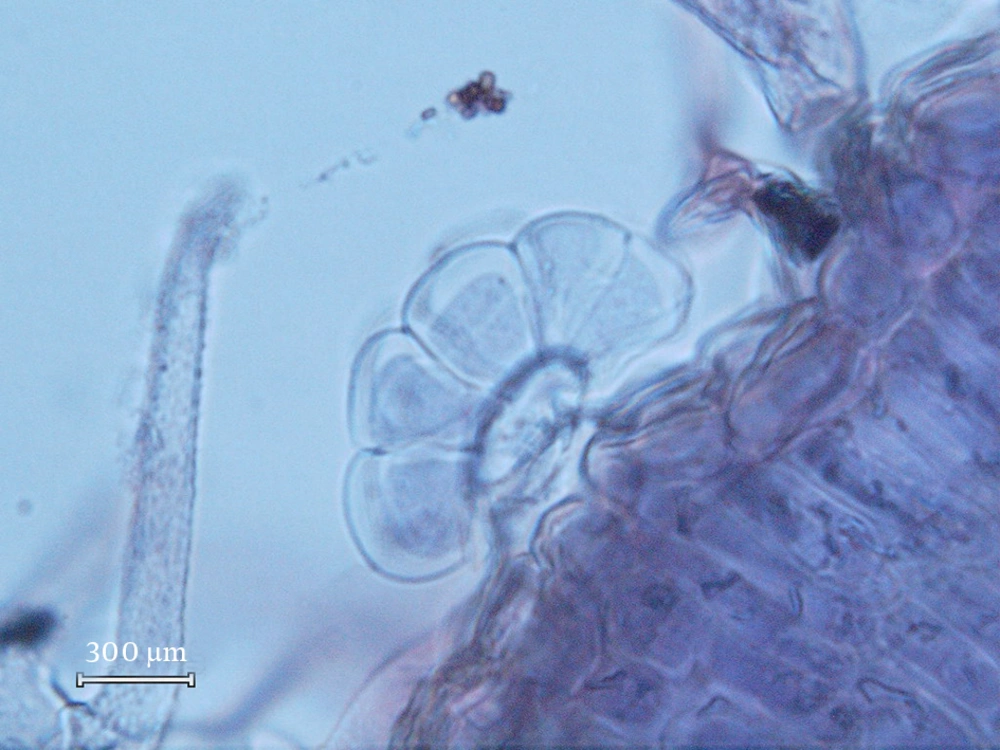
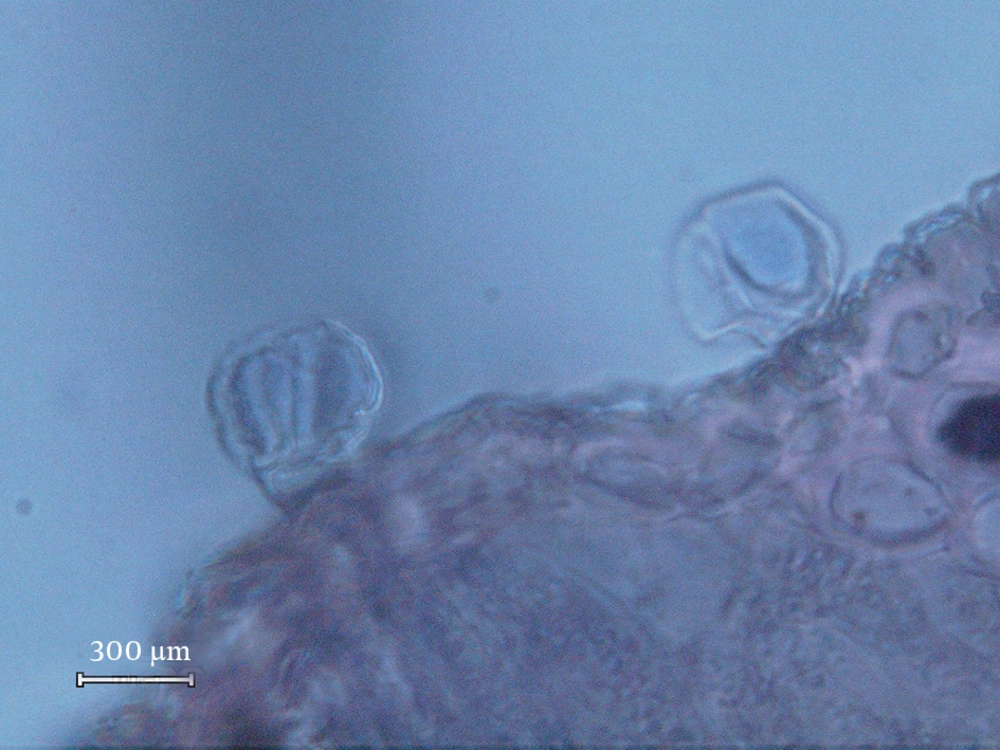
The following types of the glandular trichomes can be recognized: one capitate and two types of peltate trichomes. Capitate trichomes consisted of a basal cell, a short unicellular stalk and a bicellular secretory head (Figure 1). The peltate glandular trichomes were predominantly on the abaxial surface. They consisted of one basal epidermal cell, a unicellular stalk cell and a multi-cellular (Figure 2) or tetracellular secretory head (Figure 3). The peltate trichomes were more frequent and numerous than capitate trichomes. Analyzing the composition of volatile oils produced by the flower part (21) of the plant during the reproductive stage resulted in various compounds in different proportions. P. abrotanoides collected from Baluchistan consisted of 41 characteristic compounds, and P. abrotanoides collected from Khorasan revealed 29 compounds. The constituents of the oils are presented on Tables 1 and 2. However, the main compound of the oil were 1,8-cineole and camphor (Table 3). Viridiflora and neryl acetate were not identified with yellow glandular trichomes collected from Baluchistan, but the amount of camphor was very high on the samples collected from Khorasan. The influences of geography and environment on the plant secondary metabolites, especially essential oils were reported in many plants (2, 5). This result is similar to the published paper on flower part of P. abrotanoides collected from Khorasan Province on which the main compounds were camphor (19.08%), 1,8–cineol (18.10%), and α-pinene (9.49%) (3).
| Compound | Retention time | Kovats index | Percentage |
|---|---|---|---|
| Tricyclene | 3.62 | 927 | 0.22 |
| α-Pinene | 3.85 | 939 | 6.97 |
| Camphene | 4.10 | 954 | 2.64 |
| Isopropyl tiglate | 4.50 | 976 | 0.22 |
| β-Pinene | 4.64 | 979 | 1.6 |
| Myrcene | 4.90 | 991 | 0.74 |
| α-Phellandrene | 5.23 | 1003 | 0.29 |
| δ-3-Carene | 5.43 | 1011 | 8.63 |
| α-Terpinene | 5.51 | 1017 | 0.61 |
| 1,8-Cineole | 5.93 | 1031 | 14.41 |
| Ocimene | 6.26 | 1037 | 0.82 |
| γ-Terpinene | 6.54 | 1060 | 0.82 |
| α-Terpinolene | 7.69 | 1089 | 2.44 |
| Camphor | 8.94 | 1146 | 1.97 |
| Borneol | 9.66 | 1169 | 6.18 |
| Terpinen-4 ol | 9.93 | 1177 | 0.65 |
| para-Cymen-8 ol | 10.20 | 1183 | 0.20 |
| Fenchol | 10.38 | 1289 | 2.33 |
| Linalyl oxide acetate | 12.38 | 1289 | 1.96 |
| Bornyl acetate | 13.31 | 1289 | 3.01 |
| Terpinenyl acetate | 15.28 | 1349 | 1.95 |
| Neryl acetate | 16.44 | 1362 | 3.19 |
| β-Caryophyllen | 17.49 | 1425 | 8.18 |
| α-Humulene | 18.51 | 1455 | 8.33 |
| Alloaromadendrene | 18.63 | 1460 | 0.24 |
| Dehydroaromadendrene | 19.63 | 1463 | 0.30 |
| α-Amorphene | 20.20 | 1485 | 0.54 |
| δ-Cadinene | 20.47 | 1523 | 0.39 |
| Caryophyllene oxide | 22.16 | 1583 | 0.60 |
| Viridiflora | 22.58 | 1590 | 7.88 |
| Humulene epoxide | 22.95 | 1608 | 0.72 |
| Naphthalene 2-acetyl | 23.10 | 1609 | 0.42 |
| epi-α-Cadinol | 23.86 | 1640 | 1.91 |
| β-Eudesmol | 24.08 | 1651 | 0.50 |
| α-Eudesmol | 24.17 | 1654 | 0.93 |
| Valeranone | 24.67 | 1675 | 0.43 |
| α-Santalol | 25.60 | 1675 | 1.72 |
| Compound | Retention time | Kovats Index | Percentage |
|---|---|---|---|
| α-Pinene | 3.84 | 939 | 8.40 |
| Camphene | 4.10 | 954 | 3.91 |
| β-Pinene | 4.63 | 979 | 1.87 |
| Myrcene | 4.89 | 991 | 0.87 |
| δ-3-Carene | 5.37 | 1011 | 6.13 |
| α-Terpinene | 5.49 | 1017 | 0.37 |
| P-Cymene | 5,69 | 1025 | 0.78 |
| 1,8-cineole | 5.91 | 1031 | 15.52 |
| Ocimene | 5.99 | 1037 | 0.35 |
| γ-Terpinene | 6.52 | 1060 | 0.63 |
| Terpinolene | 7.31 | 1089 | 0.47 |
| Camphor | 9.03 | 1146 | 19.22 |
| Borneol | 9.61 | 1169 | 5.60 |
| γ-Terpineol | 10.32 | 1299 | 1.10 |
| Bornyl acetate | 13.26 | 1310 | 2.43 |
| Terpinenyl acetate | 15.25 | 1349 | 2.19 |
| α-Copaene | 16.01 | 1377 | 0.48 |
| α-Gurjunene | 17.06 | 1410 | 0.92 |
| β-Caryophyllene | 17.39 | 1425 | 5.62 |
| α-Humulene | 18.41 | 1455 | 4.85 |
| Epizonaren | 19.75 | 1502 | 0.54 |
| γ-Cadinen | 20.21 | 1514 | 2.69 |
| δ-Cadinen | 20.48 | 1523 | 1.69 |
| Caryophyllene oxide | 22.15 | 1583 | 0.61 |
| 2-Acetyl-Naphthalene | 23.08 | 1609 | 1.36 |
| epi-α-Cadinol | 23.87 | 1640 | 7.03 |
| α-Eudesmol | 24.13 | 1654 | 0.49 |
| α-Bisabolol | 24.99 | 1685 | 0.50 |
| Compound | Perovskia abrotanoides (Baluchistan) | Perovskia abrotanoides (Khorasan) |
|---|---|---|
| 1,8-Cineole | 14.41 | 15.52 |
| δ-3-Carene | 8.63 | 6.13 |
| α-Pinene | 6.97 | 8.40 |
| β-Caryophyllene | 8.18 | 5.62 |
| α-Humulene | 8.33 | 4.85 |
| Borneol | 6.18 | 5.60 |
| Camphor | 1.97 | 19.22 |
| Viridiflora | 7.88 | Trace |
| Neryl acetate | 3.91 | Trace |
| epi-α-Cadinol | 1.91 | 7.03 |
5. Discussion
It was reported that the aerial part of P. abrotanoides collected from Khorasan Province showed the components with the following pattern; cineole (32.4%), myrcene (13%), α-pinene (10.2%), camphor (9.1%), β-caryophyllene (7.9%), and α-humulene (6.4%) (4). By analogy to our results presented in Table 1, using different plant part for extraction of the oil can be the reason for variation of the constituents of the essential oil. Similar differences can be seen on another report on aerial part of P. abrotanoides collected from Sistan and Baluchistan region that showed the the following components: ocimene (13.93%), 1,8-cineole (11.22%), geranyl acetate (10.49%), β-caryophyllene (8.76%), α-humulene (6.64%), and linalool (3.26%) (20).
According to many studies, given the same species with similar part (flower) and method of distillation, but collected from different geographical regions; the most influential factor on the variation of essential oil composition is the distance between species. Therefore, the environment condition (temperature, humidity, interaction with other species, etc.) play a significant role in the composition of the oil. In addition, other factors like method of distillation and the kind of organ of plant distillation are responsible for differences between chemical compounds.
In conclusion, hydrodistillation is the best way to obtain essential oil and flowers (reproductive organs) are the best part of the plant for essential oil collection. It is well known that the presence and composition of volatile oil can be affected by several factors, including physiological variations, environmental conditions, geographical variations, genetic factors, and evolution. The structure and micromorphology in glandular trichomes of Perovskia abrotanoides Karel. collected from different areas are approximately the same. Also, the color of glandular trichomes in P. abrotanoides collected from Khorasan is yellow, but the plants collected from Baluchistan are white. In comparison with other researches, the morphology of glandular and plain trichomes has high stability, but the densities of surface trichomes are different. However, the interaction between plants and environment is too complex, so more investigations about the effects of environment on plants have to be done. We also recommend polyphasic researches that can provide more accurate information.