1. Background
Sickle cell anemia is a recessive monogenic autosomal disorder. When non-polar valine is substituted for polar glutamic acid, in the position 6 of the β-chain (β6 GluVal), the mutation in chromosome 11 of the beta globin chain occurs and can result in formation of hemoglobin S (HbS) (1). The HbS has a decreased affinity to oxygen and can elevate the concentration of deoxyHbS, and, therefore, promote the formation of sickle cells. The shape of red blood cell (RBC) is changed and, also, its membrane is damaged in this situation. Several attacks to RBC lead to irreversible sickling of red cells and cause vascular thrombosis (1, 2). Sudden death may occur in individuals with defected HbS, in specific circumstances, such as flight with low air pressure and high attitudes, hypoxia during anesthesia, dehydration and intense physical pressure (1, 2).
The diagnostic methods involve hemoglobin electrophoresis, sickling test and solubility test. Complete blood counting shows normochromic normocytic pattern, with few target cells in blood smear and normal cell blood count. Furthermore, the sickling test (using sodium metabisulfite slide), for detecting HbS in heterozygous or homozygous state, may be useful in the diagnosis of this disease (1, 3).
There is no certain cure in the treatment of this anemia. However, according to several studies, hydroxyurea has been introduced, as an efficient agent in individuals with sickle cell anemia (4). Moreover, there are other medications, such as butyrate (5), clotrimazole (6-8), magnesium salts (9), nitric oxide (10) and 5-azacytidine. However, several specific side effects have been reported with their use. For example, it is reported that 5-azacytidine may be carcinogenic (11).
In traditional medicine, the golden shower tree [Cassia fistula (C. fistula)] is known as aragvadha, meaning “disease killer” (12). In the Indian literature, Flous Plant (C. fistula) is used for the treatment of skin diseases, liver disorders, diabetes (13) and hypercholesterolemia (14). There are also other beneficial effects, such as anti-worm and antifungal activities (15). People living in rural regions consume the mature fruit pulp, for different reasons, such as purgative and to protect against heart disease and abdominal pain (12, 16). The C. fistula contains beneficial compounds, including flavonoids, anthraquinones, anthocyanidins and xanthones. This plant also contains soluble carbohydrates (sucrose, fructose and glucose) and macro-mineral elements, such as calcium and potassium (17).
2. Objectives
The aim of this study was to evaluate the inhibitory and preventive effect of C. fistula extract on sickling process of HbS, among the affected RBC in vitro.
3. Materials and Methods
In this experimental-intervention study, aqueous extract of ripped fruit of C. fistula plant (prepared from Ahvaz, Iran) was obtained, using vacuum distillation method. In this method, the plant was powdered and then, 500 mL distilled water were added, as solvent, for every 35.5 g of this powder. The container was maintained at room temperature for 72 hours and condensed by a condenser, after filtration. The condensed material was dried at 40°C (18). To prepare different dilutions of the 0.28 g dried stock extract were weighed by Kern scale and dissolved in 0.9% physiologic serum solution (Merck Pharmaceuticals, Kenilworth, NJ, USA). In this study, seven dilutions of 1:2, 1:4, 1:8, 1:16, 1:50, 1:100 and 1:200 were used. Whole blood samples were obtained and transferred into plastic tubes, with 0.2 g EDTA, from individuals referred to the laboratory for electrophoresis assay. Five samples, with normal peri-pheral blood smear morphology and positive sickling test, were selected to treat with the extract. The RBC were washed three times with Merck normal saline solution 0.9% (Merck Pharmaceuticals, Kenilworth, NJ, USA), treated with different dilutions of C. fistula extract and then incubated at 37ºC in WT-Binder incubator (Binder GmbH, Tuttlingen, Germany) for 24 hours. Accordingly, the same protocol was carried out on three normal subjects, as negative control group, and trait samples were considered, as positive control, before the treating. After 24 hours incubation time after treating RBC with C. fistula extract, the sickling test was con-ducted for every sample. To perform this test, one drop of sample and two drops of sodium metabisulfite solution 0.2 g/L (Merck Pharmaceuticals, Kenilworth, NJ, USA) were inserted on the slide. The role of sodium metabisulfite was to produce a hypoxic environment condition. Then, the slide was covered, using a cover glass, along with a certain amount of paraffin, to prevent oxygen influx. After 15 minutes, the percentage of sickling cells was estimated, using optical microscopy (×40 magnification) (Olympus, Tokyo, Japan), under laboratory condition. In order to obtain further accuracy, this test was repeated at 30, 60 and 120 minutes, respectively, and all tests of trait samples were carried out as duplicate, with 1:2 to 1:8 dilutions, and as triplicate, with 1:16 to 1:200 dilutions. In case of reducing the sickling process, the test was negative, indicating that the treatment with C. fistula extract was efficient. All tests and procedures were conducted for both the test group and control group. All data were processed with SPSS version 16 software (SPSS Inc., Chicago, IL, USA). The Wilcoxon signed ranks analysis was used to compare mean percentage of sickle cells pre- and post-treatment with C. fistula extract.
4. Results
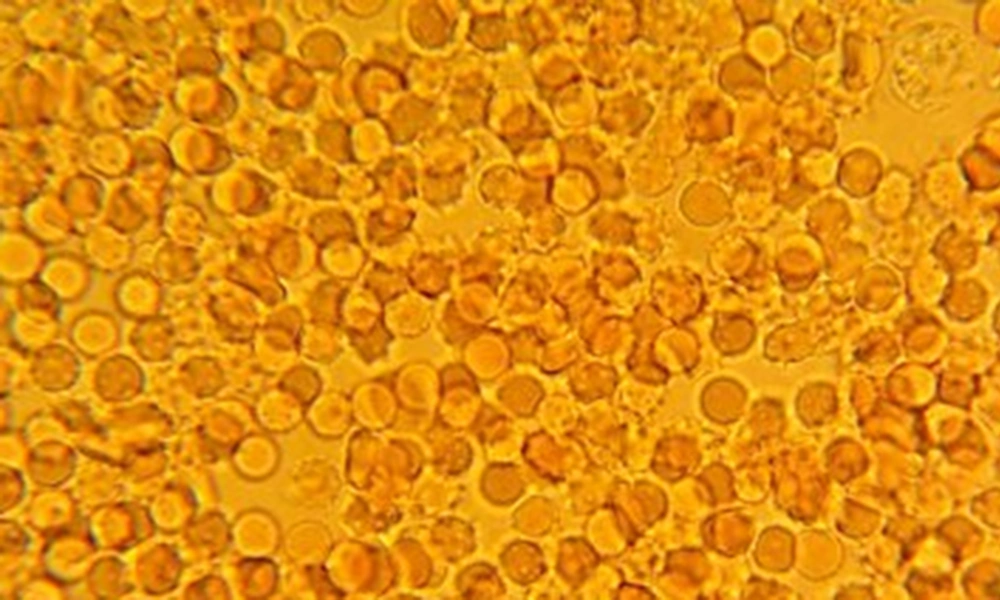
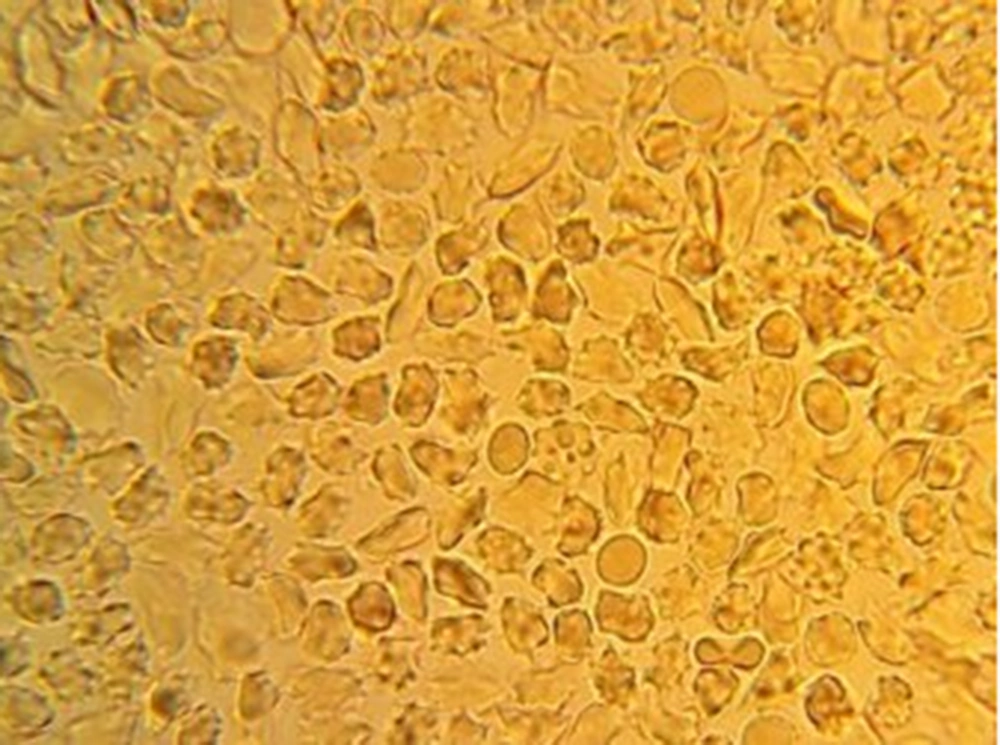
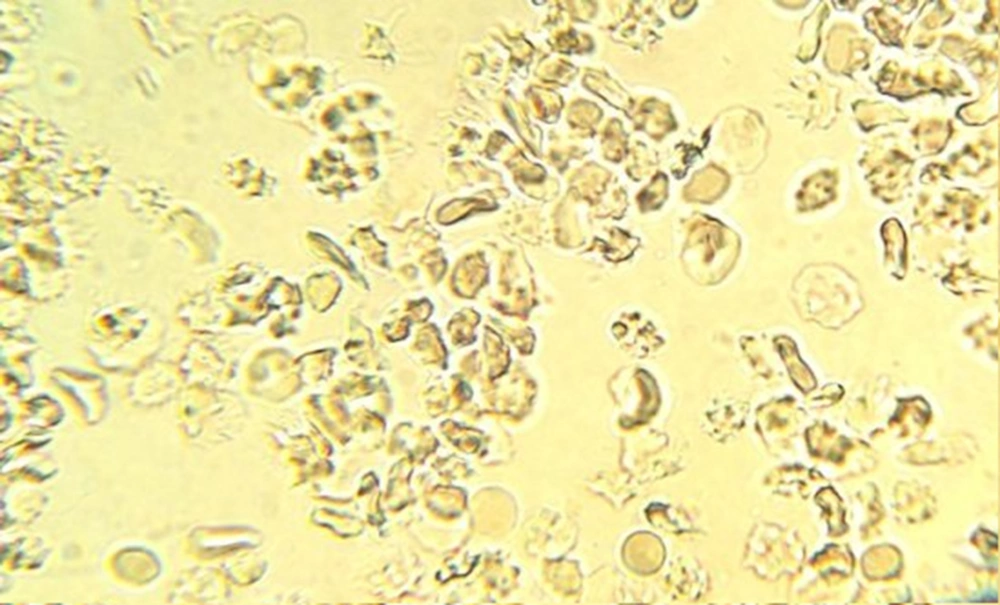
Sixty percent of trait subjects were male and 40% were female. The mean age of subjects was 17 years old, with minimum 3 years and maximum 27 years. The slide test and sickling test of the test group were normal and abnormal, respectively (Figures 1 and 2).
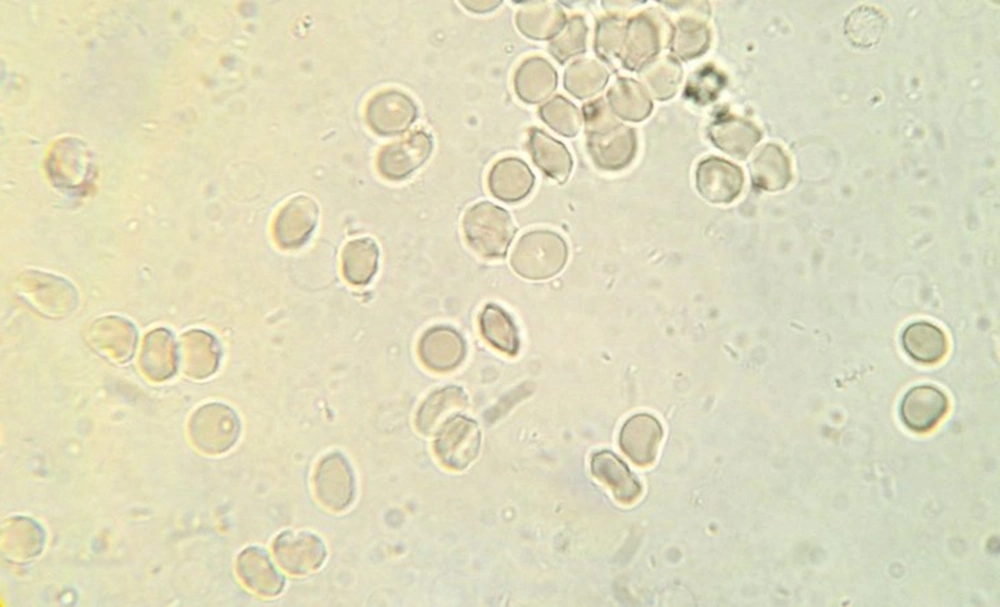
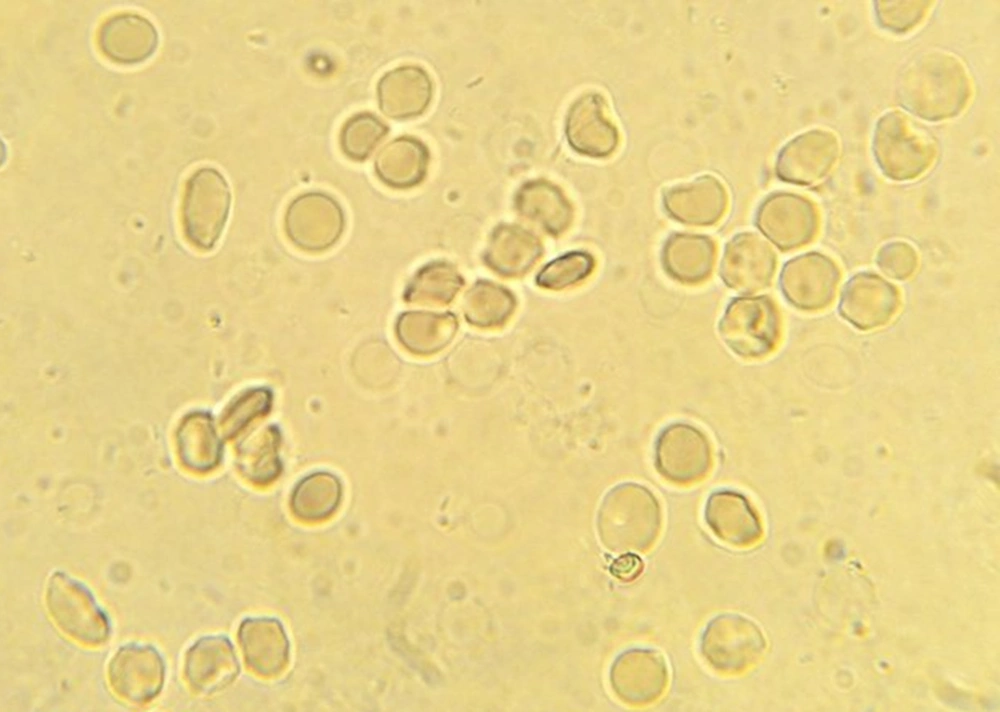
Totally, 90 sickling tests for trait samples and 21 tests for negative control samples were conducted. In 45 samples, sickling test post-treatment for 1:2, 1:4, 1:8 and 1:16 dilutions of C. fistula extract were negative, showing no sickling cells, while in dilutions of 1:50 and 1:100, the percentages of sickling cells were 20% and 80%, respectively. Moreover, in dilu-tions of 1:50, 1:100 and 1:200 the mean percentages of sickling cells were 3.78%, 25.75% and 43%, respectively, post-treatment (Figures 3 - 5). The Wilcoxon signed ranks test showed the significance of efficiency of C. fistula extract, with dilutions of 1:2 to 1:100 (P < 0.05).
5. Discussion
Aqueous extract of C. fistula prevented RBC against sickling, by 1% dilution, in vitro. Sickling attacks can lead to several complications in patients. There are different approaches to prevent these attacks, such as chemical medications. According to several studies, hydroxyurea has been known to be an efficient agent in protecting red blood cells against sickling, in patients with sickle cell anemia (4, 19, 20) Steinberg et al. showed that the intake of hydroxyurea and increased fetal hemoglobin levels, in patients with sickle cell anemia, reduced the mortality rate and frequency of continuous painful attacks and the number of neutrophils, although hemolysis was developed (4). However, treatment with hydroxyurea may not be applicable for all patients, since determination of its dosage may be difficult and its usage is expensive. The 5-azacytidine, as another chemical medication, is used for the treatment of these patients. It can increase gamma-globin synthesis (21). However, because of the carcinogenic effect of 5-azacytidine (11), its usage has been eliminated.
Furthermore, medications, such as clotrimazol and magnesium products, prevent cells from dehydration. It is indicated that intracellular fluid inhibits hemoglobin polymerization (6-9).
Chaojie Zhang et al. have suggested MX-1520, a prodrug of vanillin, with anti-sickling effect, according to the findings of their study on rats with sickle cell anemia, induced by genetic manipulation and exposed to hypoxic condition. This prodrug is converted to vanillin in vivo and, after covalent linkage to HbS, may prevent the sickling cells. This prodrug was prescribed for rats before creating a hypoxic condition. Zhang et al. used extract on rats, before induction of hypoxic condition (22). In this study, we also used the aqueous extract of the C. fistula, before hypoxic condition, this time on human cells, in vitro.
In several previous studies, on preventing the complications of sickle cell, the chemical methods were mainly investigated and traditional medicine was less studied. There were also studies introducing blood transfusions for preventing sickle attacks. In 2011, Gyang and coworkers) (23) suggested that long term and continuous transfusion of red blood cells (CTX) is an important approach to prevent cerebral infraction in patients with sickle cell anemia. Moreover, according to the findings of Wang et al. in 2005, the CTX has been recommended to improve growth in children with sickle cell anemia (24).
There are also other studies reporting the protective effects of hydroxyurea and phlebotomy against the secondary damages occurring in individuals with sickle cell anemia (25).
Regular blood transfusion, in these patients, can result in an elevation in iron levels (26). There are many disadvantages for long term blood transfusion, including alloimmunization and infection disease transmission.
It is therefore suggested to use other alternative treatments, such as C. fistula extract, which has several advantages, compared to blood transfusion. Using this extract may be cost-effective and eliminate the possible infection transmission in the blood transfusion process.
In this study, we found that the aqueous extract of C. fistula fruit protected sickling, in sodium sickling test, to a certain extent. The possible mechanism, of this action, may result from the impact of extract on the metabisulfite chemical compound and may not be associated with its effect on hemoglobin structure. Further studies are needed to investigate the mechanism, before embarking on any clinical trial, in order to prevent the complications of sickle cell anemia and enhance life expectance, in these patients.