1. Background
Infertility is one of the most common health problems in the world, which involves approximately 15% of couples. Infertility can be present in both genders, but about 50% of infertility is associated with male factor. Decreased sperm count and motility and deformity of sperm are the most important factors of male infertility (1). Various chemical drugs are available to treat infertility; however, researchers are looking for drugs with less adverse effects and toxicity. In developing countries, traditional medicine is important in maintaining health of population (2). Celery is used as food in some countries, which also has medicinal properties. On the other hand, due to adverse effects of synthetic drugs, it is necessary to conduct scientific researches to assess medicinal properties of various plants. Celery (Apium graveolens) belongs to the parsley (umbelliferae) species from the Apiaceae family, with a height of 100 cm, and a strong scent and fleshy and solid stems (3). Celery seeds contain 2% to 3% essential fat. This fat contains mostly limonene (typically 60%), selenin (10%), furocoumarin, furocoumarin glycosides, palmitic acid and federonoid (4). Celery contains vitamin C and other compounds such as phalides and coumarins, which are health-enhancing compounds. Different parts of this plant owe a wide spectrum of biological, pharmacological and therapeutic effects including anti-rheumatism, sedative, blood pressure lowering, antifungal, analgesic, anti-inflammatory, detoxification, anti-spasmodic, anti-bacterial, anti-contractions and antiepileptic (5). Recently, several studies reported increasing prevalence of infertility. Infertility can be due to consumption of natural plant compounds (phytoestrogens) if consumed in high amounts. These compounds can affect the reproductive system and reduce fertility (6). Based on available reports, plants such as celery contain phytoestrogens, which can be effective in fertility and reproductive system (7). Plasma membrane of sperm is susceptible to oxidative damage due to large amounts of unsaturated fatty acids, finally leading to decreased motility and viability of sperm. Antioxidant compounds increase sperm function and can improve fertility. Furthermore, a study indicated that celery has a protective effect on testes against sodium valproate (8) and di (2-ethylhexyl) phthalate (9). Studies demonstrated that celery protects testes from functional and structural damages and sperm from toxicity induced by atrazine (10) and quinine sulfate (11).
2. Objectives
The present study was performed to assess the effects of hydroalcoholic extract of celery leaf on the number of sperm and testes structure in male rats.
3. Materials and Methods
This experimental study was performed in Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences (AJUMS). Thirty-two male Wistar rats (weight range of 170-220 grams) were obtained from the central animal house of AJUMS. Animals were housed in plastic cages with 12/12h light/dark cycle at 21 ± 2˚C. These conditions were maintained constant throughout the experiments. The study was performed in accordance with the principles of laboratory care established by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3.1. Extraction
To prepare the extract, leaves of celery was prepared from agricultural lands of Hamidiye (Khoozestan, Iran) and specified by an expert in Pharmacognosy department of pharmacy faculty of AJUMS. The leaves were shade-dried and milled to fine powder using a mechanical grinder. Powder (50 g) was soaked in 200 mL of 70% ethanol (Merck, Germany) for three days with occasional shaking. The solution was passed through filter paper and evaporated in oven at 40˚C for 48 hours. Extracted powder was kept at 4˚C until use. Concentrations of 100 and 200 mg/kg/BW were prepared from obtained powder of celery using normal saline as solvent (Daru Pakhs, Iran) (12). Hydroalcoholic extract and solvent administrated by gavage once every two days for 60 days. The duration of spermatogenesis in Wistar rats is 52 days according to the literature (13, 14).
3.2. Experimental Design
In this study, animals were randomly divided into four groups of eight rats each (15). The groups were divided as follows: group I as control did not receive any medication; group II as sham received 1 mL solvent of normal saline; group III received hydroalcoholic extract of celery (100 mg/kg); group IV received hydroalcoholic extract of Celery (200 mg/kg) (9). At the end of the experimental, rats were anaesthetized and killed by IP injection of ketamine (60 mg/kg) and xylazine (10 mg/kg) (Alfasan, Holland). Then, testes and epididymis were carefully separated. Testes were weighed by a digital scale (Sartorius-PT120-Germany) and fixed by Bouin’s fixative, dehydrated through increasing concentrations of ethanol and embedded in paraffin. Then five sections with thickness of 5 µm (an interval of 100 µm) in each animal were stained with hematoxylin-eosin. The sections were studied regarding morphometric and histopathologic changes (10 fields in each section) (16) by Motic software (Micro-Optic Industrial Group CO., LTD., UK ) (17) and light microscope, respectively (Olympus, 3H-Z-Japan). Sperms were extracted from the caudal portion of epididymis by fragmentation of epididymis into 1 mL in normal saline. After five minutes incubation at 37˚C, sperm suspension was diluted with a ratio of 1:100. This diluted solution was used for sperm count using Neubauer’s slide. Therefore, a drop of solution was poured on the slide, and lamella was placed on it; sperms were counted by light microscope in corner areas (18).
3.3. Statistical Analysis
All data were expressed as mean ± SE. Data was analyzed by One-way ANOVA and LSD tests using SPSS for windows (version 15, IBM, USA). P < 0.05 was considered as statistically significant.
4. Results
4.1. Testis Index
There was no significant difference between the groups regarding testis index (P > 0.05) (Table 1).
| Group | Control | Sham | Experimental Group 1, 100 mg/kg | Experimental Group 2, 200 mg/kg |
|---|---|---|---|---|
| Testicular index, g | 0.63 ± 0.01 | 0.65± 0.01 | 0.66± 0.01 | 0.65± 0.01 |
a Comparison of Mean ± SE of testis index between the experimental (100 and 200 mg/kg hydro alcoholic extract of celery) and control groups.
4.2. Morphometric Studies
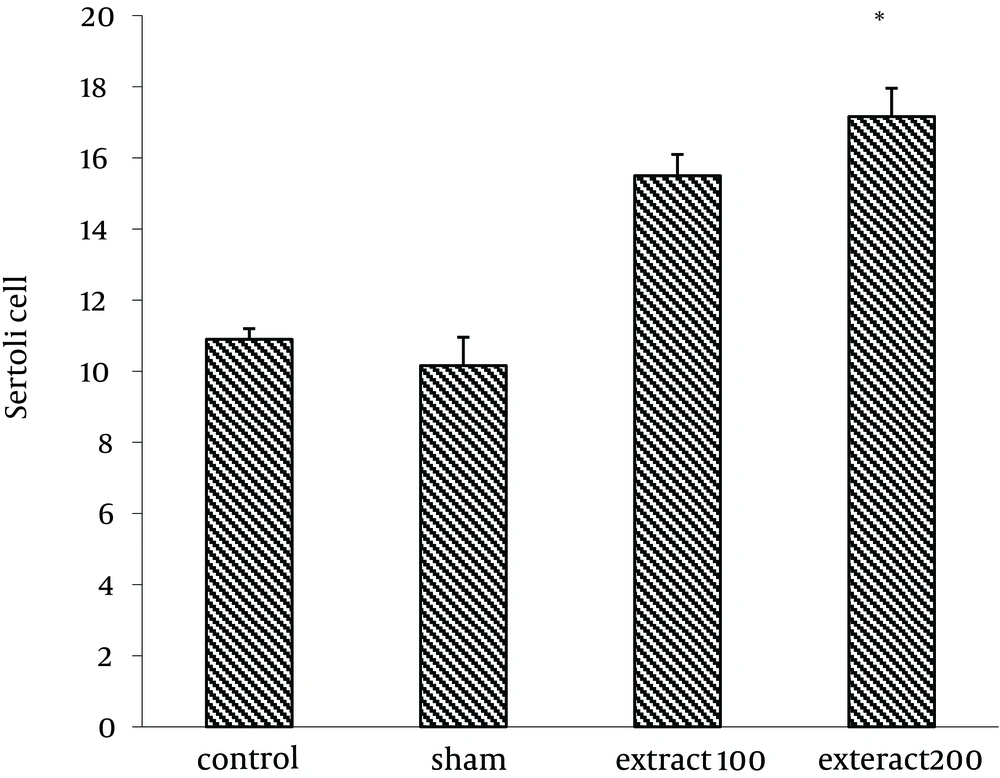
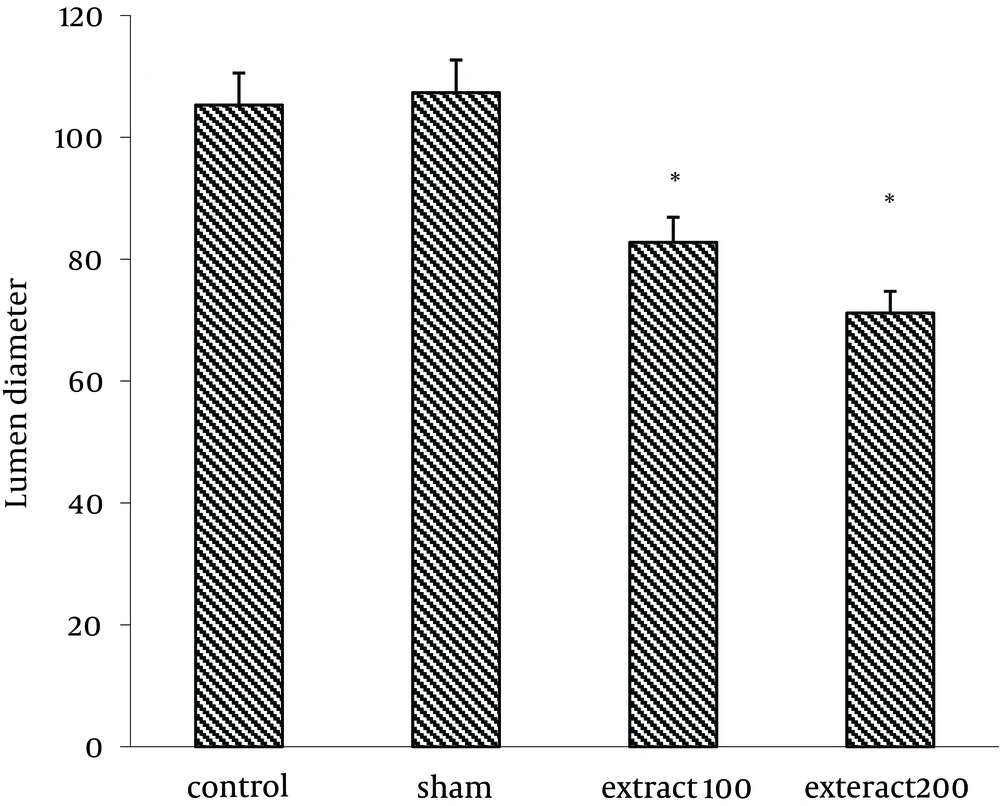
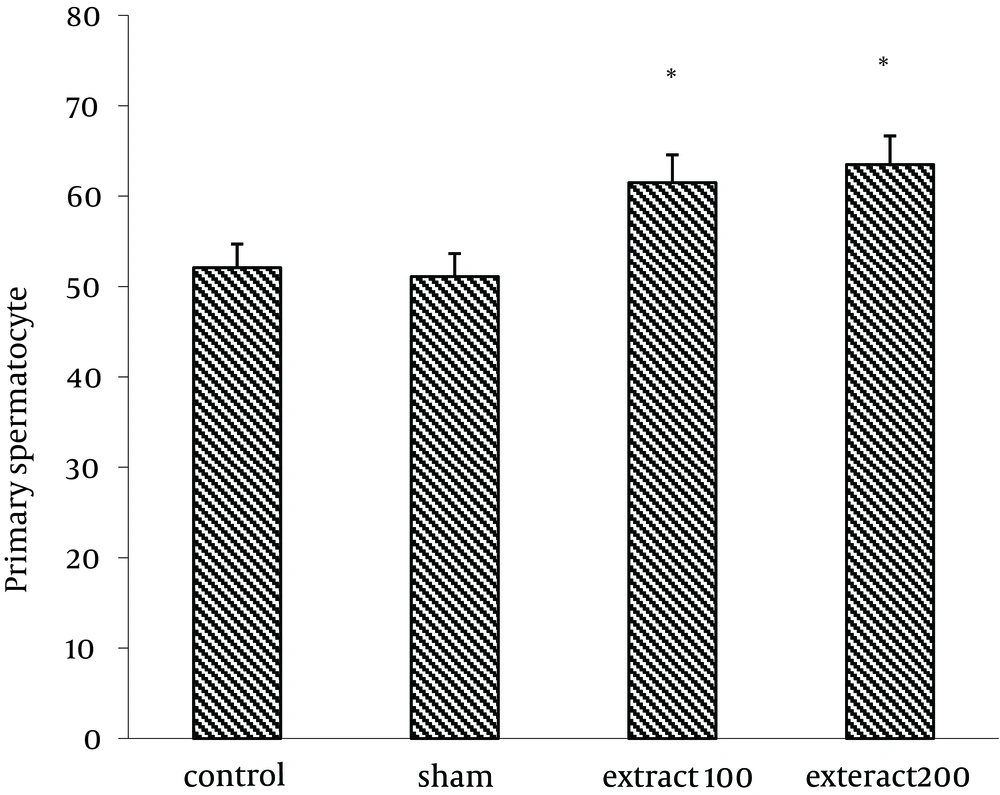
Number of Sertoli cells increased significantly in group receiving extract (200 mg/kg) (17.16 ± 0.6) compared to control group (10.99 ± 0.47) (P < 0.05) (Figure 1). Similarly, the results showed that lumen diameter was significantly reduced in groups III and IV (82.77 ± 7.03 and 71.18 ± 5.53, respectively) compared to the control group (P < 0.05) (105.3 ± 5.6) (Figure 2). The mean number of primary spermatocytes was significantly increased in groups III and IV (61.5 ± 2.5 and 63.5 ± 6.5, respectively) compared to the control group (P < 0.05) (Figure 3).
4.3. Sperm Count
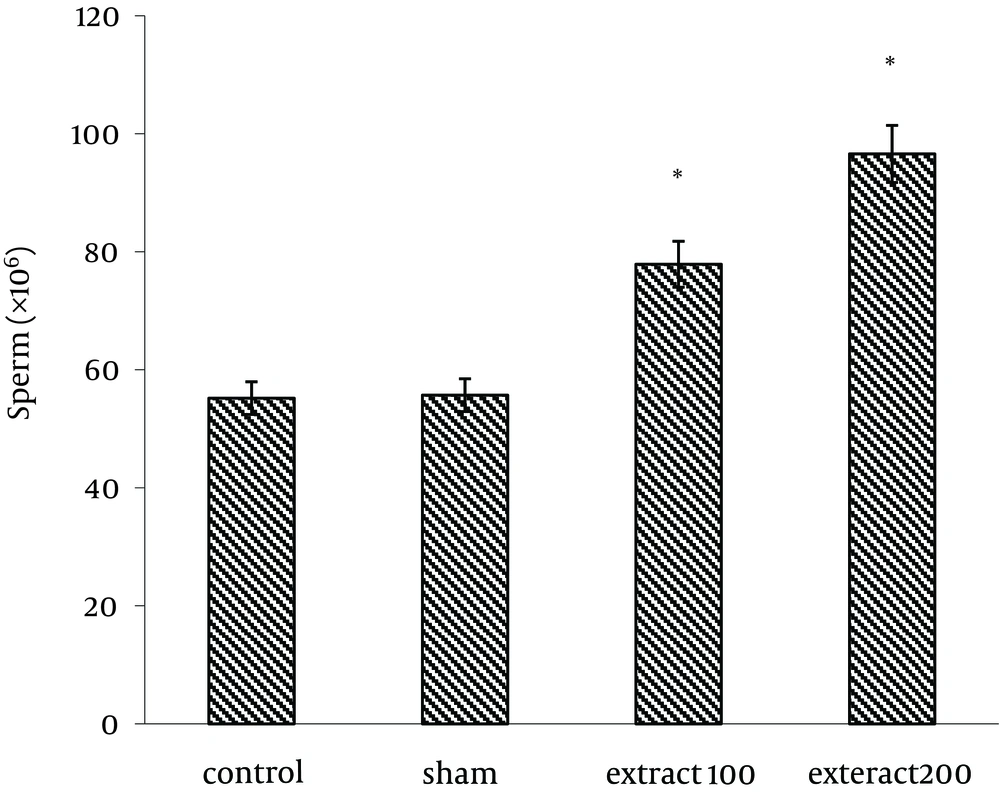
Administration of hydroalcoholic extract of celery with dosages of 100 (77.9 ± 5.8) and 200 mg/kg (89.68 ± 5.5) increased sperm count compared to the control group (56.78 ± 4.9) (P < 0.05) (Figure 4).
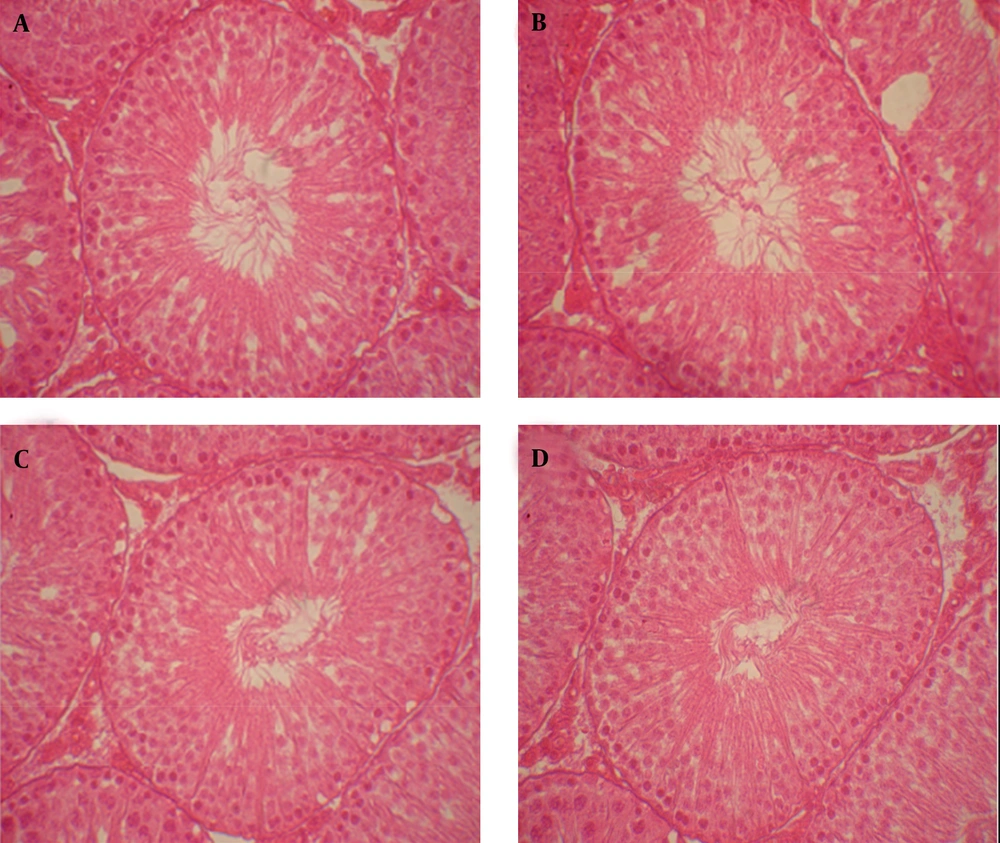
I, control; II, sham (solvent of normal saline); III and IV, experimental (100 and 200 mg/kg hydroalcoholic extracts of celery). Germinal epithelium of seminiferous tubules and interstitial tissue in the control and sham groups were normal. Germinal epithelium was thicker in groups III and IV.
4.4. Histological Study
Germinal epithelium of seminiferous tubules and interstitial tissue in the control and sham groups were normal and tissue damage was not observed (Figures 5A and B). Moreover, we did not observe any changes in arrangement and structure of epithelial cells in seminiferous tubules and interstitial tissue in groups receiving hydroalcoholic extract of celery compared to groups І and ІІ; only the germinal epithelium was thicker in these groups (Figures 5C and D).
5. Discussion
Historically, plants have been considered as a valuable source; however, they are mostly used for food consumption rather than their therapeutics effects. The current study showed that hydroalcoholic extract of celery has useful effects on spermatogenesis and testis in male rats. In this study, we observed a slightly increase of testes index in groups ІІІ and IV, but was not statistically significant. In addition, we did not observe any changes or destructive effects on testicular tissue. The results indicated that used dosages of hydroalcoholic extract of celery had no clear effect on these parameters. Celery contains flavonoids and phenolic acids with anti-inflammatory effects; also, apigenin and epiein as main flavonoids of celery owe anti-inflammatory properties (19). Apigenin as an antioxidant inhibits the production of hydrogen peroxide and IgE, which is responsible for inflammation and allergic responses (20). Apigenin has inhibitory effects on cyclooxygenase and lipoxygenase (21). Therefore, celery is able to reduce harmful effects of free radicals on cells and prevents cell death and loss of weight or tissue volume due to its antioxidant and anti-inflammatory properties. These results are in agreement with some previous investigations (9). Besides, our results indicated that Sertoli cells and primary spermatocytes increased and lumens diameter reduced in experimental groups. Our result is similar to those reported in previous studies (10, 11). A previous study suggested that hydroalcoholic extract of celery seed increased testosterone secretion (22). The level of testosterone as the most important androgenic hormone is effective in the evolution and proliferation of germ cells and spermatid differentiation. Likely, celery directly affects Sertoli cells by stimulating secretion of testosterone (23). Sertoli cells have a major role in differentiation and development of spermatogenesis. These cells promote caryokinesis karyokinesis, cytokinesis and differentiating of spermatozoa with producing growth factors such as activin in the presence of calcium ion. Furthermore, Sertoli cells secrete tubule fluid, which helps to nourish sexual cells and plays an important role in their supporting (24). In addition to the shape and quality of sex cells, blood concentration of reproductive hormones is an important parameter to evaluate function of the reproductive system. Testosterone affects seminiferous tubules and induces spermatogenesis (25). Our findings indicated that administration of hydroalcoholic extract of celery at both dosages increased sperm count. In a similar study by Hamza et al. it was shown that celery extract decreased toxic effects of sodium valproate and increased all of spermatogenic cells lineages (8). Sperms count is one of the important factors of fertility, and decrease of sperm count can decrease the probability of a successful pregnancy. Researchers suggested that spermatogenesis and maturation of sexual cells depend on protection of cytotoxic and pathologic lesions that threaten these events (26, 27). Previous studies demonstrated that induced damage by free radicals and oxidative stress could cause various disorders, such as infertility (28, 29). Spermatogenic cells, unlike other cells are very sensitive to oxidative damage, which is due to high amounts of poly-unsaturated fatty acids in their plasma membrane and very low levels of cytoplasmic antioxidants (30). Peroxidation of membrane fatty acids leads to loss of membrane fluidity and decreases its enzyme activity and ion channels leading to gradual loss of the ability of sperm to bind to the oocyte. The main effect of lipid peroxidation in all cells, especially sperm is disturbance of structure and function of organelles or cell membranes (ion transport processes, fluidity and permeability, metabolic gradients). These changes can affect the structure and function of sperm (31). In normal conditions, a balance exists between reactive species oxygen and free radicals production and antioxidant defense system. An imbalance between free radical production and antioxidant defenses leads to injuries induced by free radicals. Flavonoids are secondary metabolites of plant compounds with powerful antioxidant properties, and cannot be synthesized in the body and must be received through diet. Celery is a strong antioxidant due to flavonoids such as apiein and apigenin (32, 33). Antioxidant compounds are able to protect cell membranes against damage (34). Antioxidants affect hypothalamic-pituitary-testicular axis directly or indirectly, thus increasing sperm count and fertility (35, 36). Moreover this herb contains vitamins E and C (32, 33). In an experimental study, it was shown that these vitamins improve sperm parameters such as count and motility (37, 38). Vitamin E is a powerful antioxidant with a supporting role in quality and quantity of sperm, fertilization and fertility in humans. This vitamin is abundant in Sertoli cells, spermatogonia, and round spermatid. Therefore, increasing sexual cells due to protective effect of vitamins and compounds of celery is justified leading to reduction in diameter of seminiferous tubules lumen. The results of the present study confirmed that hydroalcoholic extract of celery leaf could increase spermatogenesis in rats. This increase was more pronounced at higher dosage. Perhaps this plant could be used to treat infertility in men. However, it is suggested to perform further experimental and clinical studies on total extracts of this plant and its exact mechanisms on spermatogenesis.