1. Background
Melanin is a pigment with a polymeric complicated structure, which plays an important role in determination of mammalian skin and hair color. It is widely distributed in organisms, including fungi, plants and animals. Another role of melanin is absorption of Ultraviolet (UV) rays to protect the skin (1). Melanin synthesis occurs in a special kind of skin cells named melanocytes, which is stimulated by UV radiations (2). Keratinocytes are another kind of cells that surround melanocytes. Melanin is transported to keratinocytes after its synthesis (3).
Melanogenesis is a process that results in melanin production. This process is a combination of two kinds of enzymatic and chemical reactions. The enzymatic reactions are catalyzed by tyrosinase, which oxides L-tyrosine to dopaquinone. This step is a rate-limiting step in melanogenesis (4). Tyrosinase is a key enzyme that catalyses hydroxylation of monophenols to O-diphenols (monophenolase activity) and oxidation of O-diphenols to quinones (diphenolase activity) (5). Next reactions occur spontaneously at physiological pH value (4).
Abnormal accumulation of melanin leads to hyperpigmentation disorders. The enzymatic browning of vegetables and fruits is another issue that tyrosinase represents (4). Tyrosinase could contribute to neurodegenerative diseases such as Parkinson through formation of the neurotoxin 5-S-cysteinyl-dopamine during the oxidation of dopamine to an O-quinone and reaction with cellular thiols (6).
These undesirable phenomena encouraged researchers to find new tyrosinase inhibitors for use in cosmetic, medicine and food industries to prevent or treat hyperpigmentation disorders and food browning (4). The common tyrosinase inhibitors such as hydroquinone regardless of potent tyrosinase inhibitory effect, have limitations in use, because of its mutagenicity and cytotoxicity effects (7). Also very few tyrosinase inhibitors are used in food industry due to off-flavors, off-odors, food safety and economic values (1). Furthermore, these adverse effects spurred us to find natural tyrosinase inhibitors.
It has been shown that Physalis edulis L., Marrubium velutinum L., Marrubium cylleneum L., Althea officinalis L. and Cuminum cyminum L. could inhibit tyrosinase (8-10). Since close plants and other species of a genus may have the same effects, we selected physalisalkekengi, Marrubium vulgare L., Alcea rosea L. and Bunium persicum B. Fedtsch for this study.
P. alkekengi (Solanaceae), known as winter cherry, is an indigenous plant to central and southern Europe, China and Indochina and naturalized in the U.S (11). It has antimicrobial (12), antioxidant (13), hypoglycemic (14), antineoplastic (15), anti-inflammatory (16), antispasmodic (17) and anti-fertility (18) effects. Winter cherry in folk medicine is used as a diuretic in kidney and bladder ailments and in the treatment of gout and rheumatism (11).
B. persicum (Apiaceae) is distributed in central Asia, Iran, Pakistan, Afghanistan, Keshmir and Pamir (19). The B. persicum exhibits antimicrobial (20), antifungal (21), antioxidant (22), α-amylase inhibitory (23), anticonvulsant (24), antinociceptive, anti-inflammatory (25) and antihistaminic (26) effects.
A. rosea (Malvaceae), known as Hollyhock is widely grown in gardens and parks in the Southern Europe and Asia. Some pharmacological effects including antibacterial, analgesic, anti-inflammatory and cytotoxic activities (27-29) have been reported.
M. vulgare (Lamiaceae) is a medicinal plant used in folk medicine for many diseases including acute and chronic bronchitis, whooping cough, asthma, tuberculosis, pulmonary catarrh, respiratory infections, diarrhea, jaundice, debility and painful menstruation (11). It has antibacterial, antifungal (30), antioxidant (31), analgesic (32), anti-diabetic (33), antihepatotoxic (34), hypotensive (35), antispasmodic (36) and antinociceptive (37) activities.
2. Objectives
P. alkekengi, M. vulgare, A. rosea and B. persicum are some plants available in Iran, which have not been subjected for tyrosinase inhibitory effects before, so the aim of this study was to evaluate inhibitory effects of these plants on mushroom tyrosinase activity.
3. Materials and Methods
3.1. Chemicals
Mushroom tyrosinase (lyophilized powder, 3130 unit/mg solid) and L-dopa (powder, ≥ 98 %) from Sigma Chemical Co, kojic acid (powder, ≥ 98.0 %) from Fluka Chemical Co, DMSO (Dimethyl sulfoxide), ethanol and n-hexane from Sigma Chemical Co were used.
3.2. Plant Materials
P. alkekengi (from sites near Tehran), A. rosea (from Isfahan), B. persicum (from Chubar Mountains in Kerman) and M. vulgare (from 1000 m above the sea level near Tehran) were collected. They were identified by the pharmacognosy department of Ahvaz Jundishapur University of Medical Sciences. Then the plants were shade dried at room temperature and milled.
3.3. Preparation of Extracts
The aerial parts of P. alkekengi, A. rosea and M. vulgare and seeds of B. persicum were extracted with ethanol 80% for 72 hours. After filtering, the B. persicum extract was defatted with n-hexane. The extracts were concentrated by rotary evaporator (Heidolph, Germany). Then the concentrated extracts were freeze-dried by vacuum freeze dryer (Operon, Korea) and the yields were 7.76, 12.35, 10.56, 6.69 and 8.62% for P. alkekengi, A. rosea, B. persicum (total), B. persicum (defatted) and M. vulgare, respectively. The obtained extracts powder (0.1 g) was dissolved in three mL of DMSO. Then the extracts were diluted with 25 mM phosphate Buffer (pH 6.8).
3.4. Tyrosinase Inhibition Assay
The tyrosinase activity was measured according to Karioti et al. (8) with some modifications. First, 50 µL of each extracts in six different concentrations (8.3, 4.2, 2.1, 1.0, 0.52 and 0.26 mg/mL) was mixed with 100 µL of mushroom tyrosinase (9.63 U/mL) and incubated at 25°C for five minutes. Then 100 µL of 5 mM of L-Dopa solution (substrate for tyrosinase) was added to the mixture reaction. The amount of dopachrome as a product was immediately measured at 475 nm of optical density.
The increased absorbance at 475 nm was recorded during 35 minutes. DMSO and kojic acid were used as negative and positive controls, respectively. The concentrations 0.04, 0.08, 0.12 and 0.16 mg/mL of kojic acid were used. The inhibitory effects of test samples on the enzyme activity were expressed as percent inhibition and IC50 values were calculated. IC50 value is the concentration of the inhibitor that inhibits 50% of tyrosinase activity under experimental conditions and obtained through plot of inhibition percent versus Log conc (mg/mL).

A: optical density of L-Dopa, DMSO and enzyme.
B: optical density of L-Dopa and DMSO.
C: optical density of test sample (kojic acid and the extracts), enzyme and L-Dopa.
D: optical density of test sample (kojic acid and the extracts) and L-Dopa.
3.5. Determination of Enzyme Kinetic Parameters
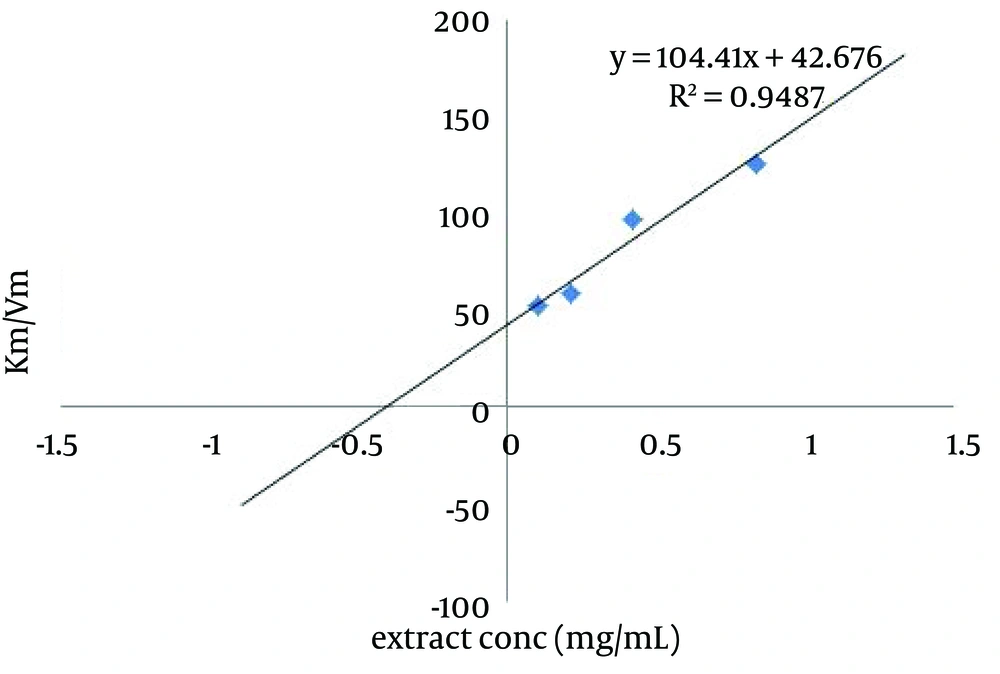
Fifty microliters of different concentrations (0.52, 1.04, 2.08 and 4.16 mg/mL) of extracts were incubated with 100 µL of mushroom tyrosinase at 25°C for five minutes. Then different volumes (100, 80, 60, 40, 20 and 10 µL) of 5 mM L-Dopa in phosphate buffer (pH 6.8) were added to the reaction mixture. Kinetic parameters, Km and Vm of tyrosinase activity were calculated by linear regression from Lineweaver–Burk plots. The inhibition constant (Ki) of an inhibitor was obtained from the secondary plot of Lineweaver–Burk plots. The secondary plot consists of vertical axis that is the slops of Lineweaver–Burk plots and horizontal axis that is inhibitor concentrations. The intercept on the horizontal axis is Ki (38).
3.6. Statistical Analysis
The measurements were performed in triplicate and mean was used in calculations.
4. Results
4.1. Inhibitory Effects of Plants on Mushroom Tyrosinase Activity
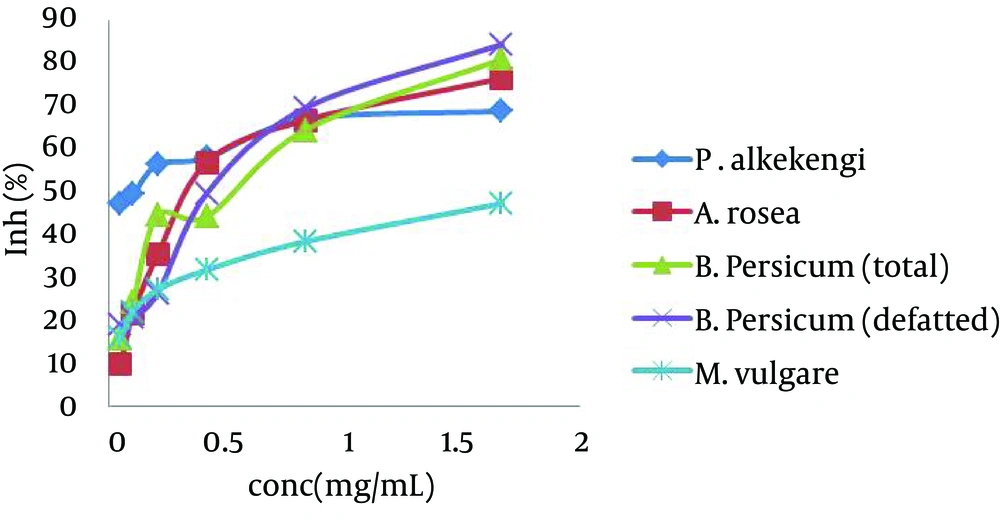
In this study, tyrosinase inhibitory effects of P. alkekengi, A. rosea, B. persicum and M. vulgare on diphenolase activity of mushroom tyrosinase were evaluated. As with increasing the concentration of all plants, the activity of tyrosinase decreased. Therefore, plants had a dose-dependent inhibitory effect on tyrosinase (Figure 1).
All total extracts of plants inhibited tyrosinase weaker than kojic acid (IC50 = 0.014 mg/mL). IC50 values of P. alkekengi, A. rosea, total and defatted extracts of B. persicum and M. vulgare were calculated 0.09, 0.38, 0.37, 0.38 and 2.82 mg/mL, respectively.
4.2. Kinetic Study of Mushroom Tyrosinase Activity
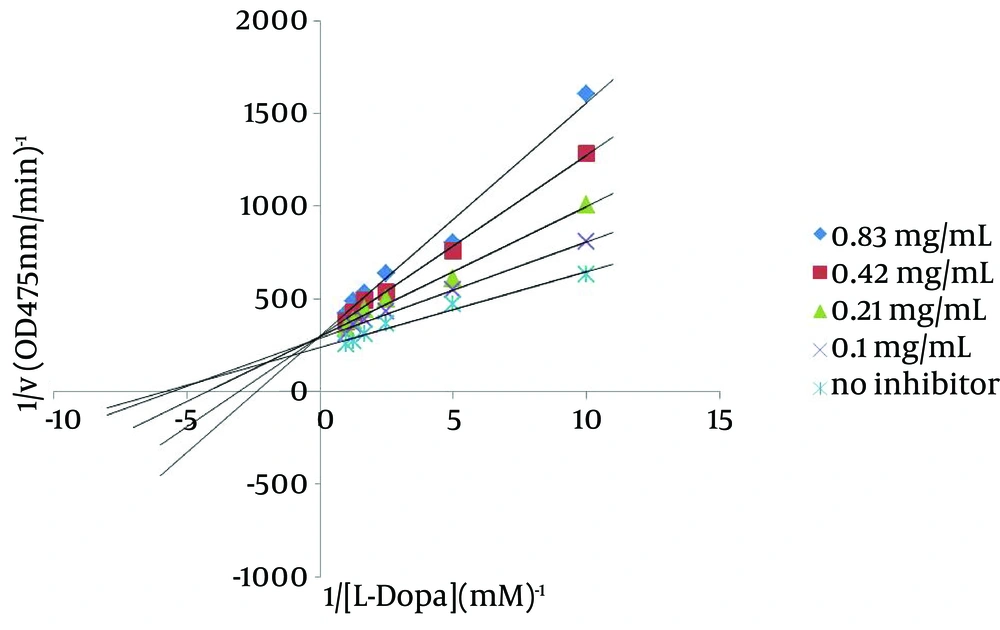
The kinetic behavior of the samples for the inhibition of tyrosinase was also analyzed by lineweaver-Burk plots. kojic acid, P. alkekengi, A. rosea and B. persicum increased the Km, but the Km was decreased when M. vulgare was used. All the extracts and kojic acid decreased Vm value (Table 1). Lineweaver–Burk plots of P. alkekengi different concentrations were shown in Figure 2. As P. alkekengi exhibited the most tyrosinase inhibitory activity, its Ki was calculated as 0.41 mg/mL (Figure 3).
| Variable | No Inhibitor | Kojic Acid | P. alkekengi | A. rosea | B. persicum (Total) | B. persicum (Defatted) | M. Vulgare |
|---|---|---|---|---|---|---|---|
| Km (mM) | 0.16 | 0.45 | 0.22 | 0.3 | 0.17 | 0.19 | 0.11 |
| Vm (U) a | 4.3 | 2 | 1.5 | 2.2 | 1.6 | 1.6 | 2.1 |
a Unite: One unit (U) of enzymatic activity was defined as the amount of enzyme needed for increasing 0.001 unit of absorbance per min at 475 nm under the experimental conditions.
5. Discussion
The tyrosinase inhibitors could be used in medicine, cosmetic and food industries to treat or prevent hyperpigmentation disorders and food browning. Based on our best knowledge, studies on anti-tyrosinase effect of A. rosea, B. persicum and M. vulgare have not been performed before.
Among test plants, P. alkekengi showed the most tyrosinase inhibitory effects, indicating its potent tyrosinase inhibitors or high content of active compounds with tyrosinase inhibitory effects.
Other species of this genus, P. divaricata and P. edulis exhibited tyrosinase inhibitory activities. P. divaricata showed tyrosinase inhibitory effect with IC50 value of 3.34 mg/mL (39) and P. edulis inhibited tyrosinase 32% at 10 mg/mL (9), while P. alkekengi showed tyrosinase inhibitory activity of 32% at 0.03 mg/mL. Therefore, P. alkekengi showed more potent tyrosinase inhibitory activity than P. edulis and P. divaricata.
The fruits of P. alkekengi have a lot of ascorbic acid (40). Ascorbic acid can prevent enzymatic reactions of tyrosinase through trapping the O-dopaquinone intermediate (1). Steroids can inhibit tyrosinase and so far three steroids from the aerial parts of Trifolium balansae were isolated which showed potent tyrosinase inhibitory effects (41). Some steroidal compounds such as physalin were isolated from P. alkekengi (42), which may contribute to its tyrosinase inhibitory activity.
Total and defatted extracts of B. persicum exhibited similar inhibitory effects on mushroom tyrosinase, which shows that non-polar compounds of B. persicum do not contribute to its tyrosinase inhibitory effect. In another study, B. persicum showed 42% inhibition at 1.14 mg/mL for diphenolase activity of mushroom tyrosinase (43). This difference could be due to various experimental conditions.
Cuminaldehyde, kaempferol, caffeic acid and p-coumaric acid are found in B. persicum (44) and can inhibit mushroom tyrosinase (1, 45-47). The tyrosinase inhibitory effect of B. persicum may be related to these compounds.
C. cyminum another kind of cumin could inhibit tyrosinase more potent than B. persicum, 45.8% at 50 µg/mL (10). It may contribute to more content of Cuminaldehyde in C. cyminum than B. persicum (44, 48). Gholamhoseinian et al. reported that C. cyminum had more anti-tyrosinase effect than B. persicum (43).
A. officinalis, another species of Althea, inhibited tyrosinase activity 48% (9) at 10 mg/mL, when A. rosea showed the same inhibition at 1.75 mg/mL. Therefore, A. rosea was more potent than A. officinalis in tyrosinase inhibition.
The flowers of A. rosea are the source of polyphenolic compounds such as anthocyanins (49) shown to have tyrosinase inhibitory activity (4). Other species of Marrubium, M. cylleneum and M. velutinum inhibited tyrosinase by 35.44% and 30.56% at 0.033 mg/mL, respectively and M. vulgare showed 16% inhibition at 0.05 mg/mL (8). Therefore, M. vulgare was weaker than M. velutinum and M. cylleneum in tyrosinase inhibition, which may be related to their high content of tyrosinase inhibitors. Amongst 45 isolated compounds with tyrosinase inhibitory activity from M. velutinum and M. cylleneum, quercetin showed the most activity (49.67% at 0.051 mM) (8). Some of these compounds such as several flavonoids (ladanein, glycoside derivatives of luteolin, chrysoeriol and apigenin) and cinnamic acid derivatives were isolated from M. vulgare (8, 33) that may contribute to its inhibitory activity.
Kojic acid, P. alkekengi, A. rosea and B. persicum exhibited mixed inhibition. They can bind to free enzyme and enzyme-substrate complex and reduce the affinity of substrate for the mushroom tyrosinase (50). However, M. vulgare showed uncompetitive inhibition. It can bind to enzyme-substrate complex and produce deactivated ESI complex and cannot bind to free enzyme (50). Although B. persicum inhibited tyrosinase through mixed mechanism, cuminaldehyde showed uncompetitive inhibition in previous studies (1).
In this study, we used crude extracts. Active compounds have a key role in inhibitory effect on mushroom tyrosinase. We did not isolate, identify and study pure active compounds. It can be performed on most potent herbs in future. According to this study, the extract of P. alkekengi had the most inhibitory effect on mushroom tyrosinase, but in vivo and clinical studies are needed to confirm the inhibitory effect and safety of P. alkekengi. Study active compounds of P. alkekengi could lead to development of new and effective tyrosinase inhibitors.
Antibacterial, antioxidant and anti-tyrosinase effects have been shown for P. alkekengi, A. rosea, B. persicum and M. vulgare; therefore, these plants could be considered as good food additives to prevent food browning and growth of microbes, but more studies on anti-browning effects and safety of these plants should be performed.