1. Background
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage (1), and a significant public health problem. There is no doubt that pain acts as a warning signal against disturbances either in the body or in the external environment of an individual. The principal objective of pain treatment is to remove or abolish the cause of pain. However, it is not always possible to do so; hence, analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs), opioids and antidepressant (2), are used for the symptomatic treatment of pain. It is believed that current analgesic drugs such as opiates and NSAIDs are not useful in all cases, because of their side effects and low potency. As a result, searching for other alternatives seems necessary and beneficial. Medicinal plants are important sources of new chemical substances that potentially have strong therapeutic effects. The genus Glaucium belongs to one of the most important alkaloid-containing families, Papaveraceae, which contains isoquinoline alkaloids including aporphines, protopines, protoberberines and proaporphines (3). This genus comprises of about 25 species of annual, biennial or perennial herbaceous flowering plants in the world (4). Glaucium has eleven species in Iran of which three, including G. vitellinum, are endemic (5). Some reports on phytochemical analyses of this genus can be found in the literature. They are rich in secondary metabolites and yield alkaloids. It is well known that occurrence of aporphinoids is a characteristic feature of this genus (6). These alkaloids have been used for a long time in traditional medicine for the treatment of various diseases, from benign syndromes to more severe illnesses. They possess a wide variety of pharmacological effects, including anti-platelet, antioxidative, antitussive, antiparkinsonian, hypotensive, anti-viral, anti-bacterial, and cytotoxic activities (7). Glaucium vitellinum is a perennial plant, which blooms from May to July. The four petals are yellow with a dark spot on the base (8, 9). Glaucium vitellinum is locally called “Shaghayegh Tamashaee” and used in Iranian traditional medicine as a laxative, sedative, anti-diabetic and anti-dermatitis (10). Glaucium vitellinum has shown to contain four major alkaloids, including dicentrine (0.8%), bulbocapnine (0.4%), protopine (0.35%), salutaridine (0.2%) and twelve minor alkaloids, including chelidonine, glaucine, corydine, isocorydine, N-methyllindcarpine, neolitsine, α-allocryptopine, N-methyllaurotetanine, dehydrodicentrine, dicentrinone , dihydrosanguinarine and dihydrochelerythrine (11). Glaucine shows analgesic and anti-inflammatory activity; tetrahydropalmatine has analgesic activities as well (12).
2. Objectives
Due to the widespread use of G. vitellinum in Iranian folk medicine for relief and treatment of pain and inflammation, we aimed to evaluate the antinociceptive activities of the methanol extract of its flowering aerial parts and to investigate the pharmacological basis for its folkloric use as a pain killer.
3. Materials and Methods
3.1. Plant Collection and Preparation of the Extract
Fresh flowering aerial parts of G. vitellinum were collected during May 2012 from the mountain areas of Khansar County, Isfahan province, Iran: (33°15′ N 50°20′ E, 2600 m). The specimens with identification vouchers were deposited in the herbarium of the Pharmaceutical Sciences Branch of Islamic Azad University (IAU), Tehran, under code number 681. The plant supply (500 g) was dried in exposure to air and away from sun light, and after being crushed, was taken to the percolator, where it was percolated by means of methanol (three times per day, 20 cc solvent each time, for 10 days). The extraction was repeated for three times. The extract was concentrated by rotary evaporator apparatus and the solvent was removed to produce a dark green gummy solid. The resultant ethanol extract was preserved in closed and dark containers in the refrigerator until use in the experiments.
3.2. Chemicals
Formalin, morphine sulfate and naloxone were purchased from Merck Chemical Company (Germany), Temad Pharmaceutical Co. (Iran) and Darou Pakhsh Pharmaceutical Co. (Iran), respectively.
3.3. Experimental Animals
In this experimental study, 96 male albino mice (20 - 25 g) were housed in groups of six to eight and were allowed free access to food and water except for the short duration of time when the animals were removed from their cages for testing. All experiments were conducted during the time between 10 AM and 1 PM with normal room light (12 hours of regular light/dark cycle) and temperature of 22 ± 1°C. The procedures were carried out in accordance with the institutional guidelines for animal care and use (ethical approval number: 22510303922063). Each mouse was used only once.
3.4. Formalin Test
Formalin test (13) was used to investigate the analgesic effect of the Glaucium vitellinum extract. Fifteen minutes after injection of different doses of the extract, 2 mg/kg of morphine or saline and 25 microliter of 0.5% formalin were injected into the right hind paw of mice and the animal was immediately placed in the formalin test container. Scoring of nociceptive behaviors began immediately after formalin injection and was continued for 60 minutes. A nociceptive score was recorded for each five-minute time block by measuring the amount of time spent in each of the following behavioral categories: 0, the injected paw was not favored; 1, the injected paw had little or no weight placed on it; 2, the injected paw was elevated and was not in contact with any surface; and 3, the injected paw was licked, bitten or shaken. A weighted average nociceptive score (pain rating), ranging from zero to three, was calculated by multiplying the time spent in each category by the category weight, summing the products, and then dividing by the total time for each five-minute time block. Individual time course determinations in the formalin test were converted to area-under-the-curve values, zero to fifteen minutes after formalin injection (AUC phase І) and 15 - 60 minutes after formalin injection (AUC phase ІІ).
3.5. Hot-Plate Test
Male albino mice (20 - 25 g) were divided to six groups (six to eight animals in each group). Control animals received normal saline (10 ml/kg, IP), while the standard group received morphine (5 mg/kg, IP) and the test groups received methanolic extract (80, 160, 200 and 250 mg/kg, IP). Animals were individually placed on a thermostatically controlled hot-plate (Borj Sanat, Iran) maintained at 55 ± 0.5°C. Briefly, the animals were placed on the hot-plate apparatus and the time between placement of the mouse on the platform and shaking or licking of the paws or jumping was recorded as the reaction time or latency of the pain response. In order to avoid damage to the paws of the animals, the time standing on the plate was limited to 15 seconds (cut-off time). Before treatment, the reaction time was taken once. The hot plate test was performed on all animals individually, 15, 30, 45 and 60 minutes after treatment.
3.6. Statistical Analysis
Comparisons between AUC phase І and phase ІІ of the formalin test in groups were made by one-way ANOVA analysis followed by the post-hoc Tukey’s test. Data obtained for pain behavior in the hot plate test were also scrutinized using one way analysis of variance (ANOVA) followed by the post-hoc Tukey’s test. The results are expressed as Mean ± Standard deviation and P < 0.05 was considered as significant difference of the means. The data were analyzed using the SPSS statistical software.
4. Results
4.1. Formalin Test
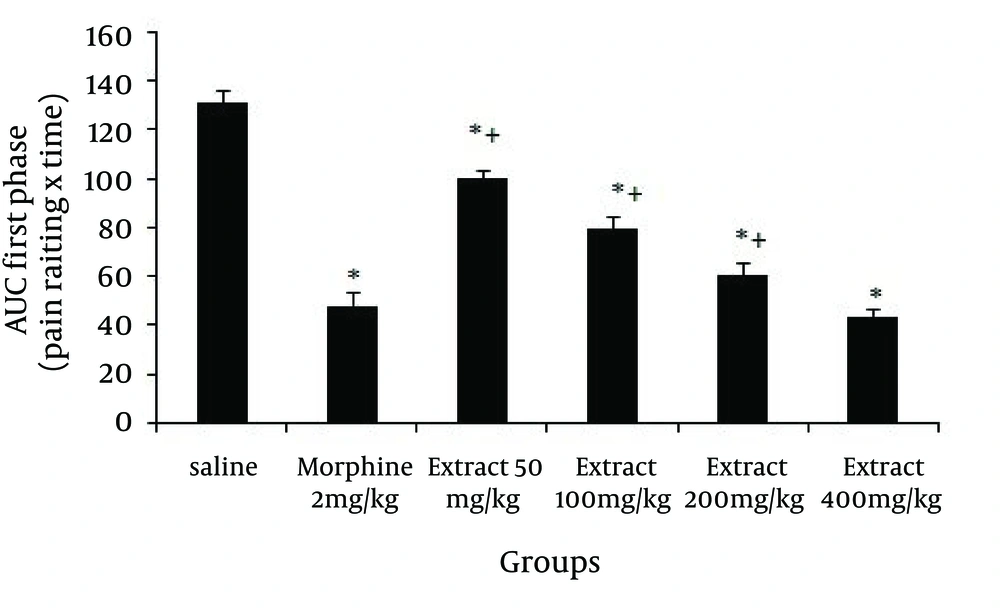
The effect of systemic intraperitoneal (IP) administration of different doses of the Glaucium vitellinum extract on the behavioral responses during the first and second phase of the formalin test was observed. As mentioned in Figure 1, in the first phase of the formalin test 50, 100, 200 and 400 mg/kg doses of the extract reduced the behavioral noxious response (P = 0.001), and morphine, as a standard analgesic drug, significantly (P = 0.001) reduced pain behavior.
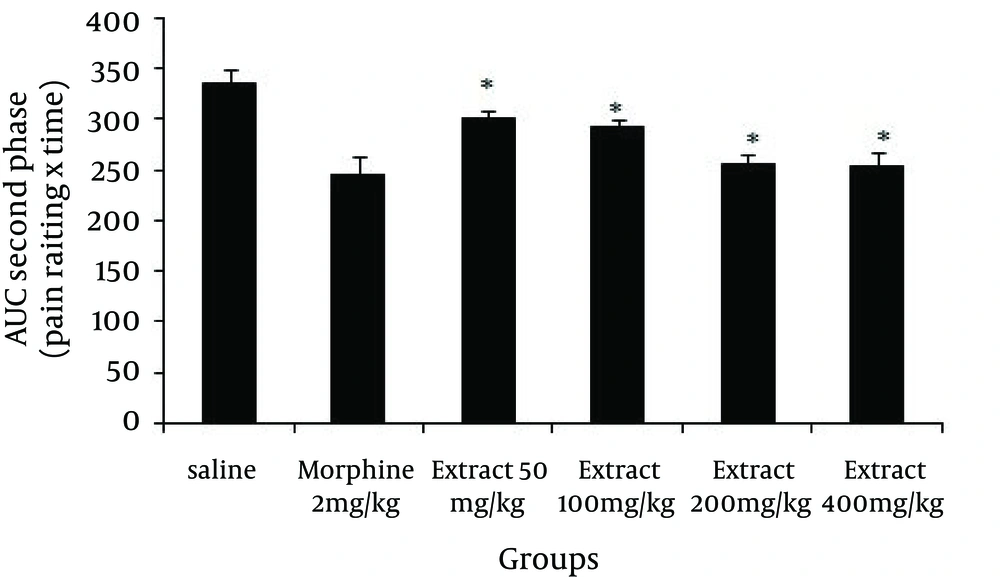
Again in the second phase of the formalin test, as indicated by Figure 2, all the studied doses of the extract reduced the behavioral noxious response (Doses of 50 (P = 0.001), 100 (P = 0.001), 200 (P = 0.001) and 400 mg/kg (P = 0.001) compared to saline), and morphine, as a standard analgesic drug, significantly (P = 0.001) reduced pain behavior.
4.2. Involvement of Opioid Receptors
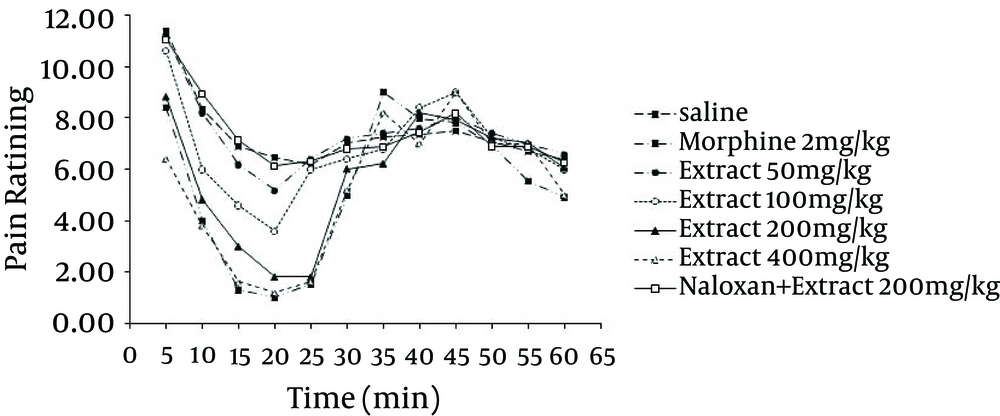
In order to investigate the involvement of the opioidergic system in anti-nociception induced by the methanol extract, groups of mice were pre-treated with non-selective opioid receptor antagonist, naloxone (2 mg/kg, IP), which was injected 20 minutes before administration of the extract (200 mg/kg) and tested using the formalin test. Pre-treatment with naloxone abolished the anti-nociceptive effect of G. vitellinum methanol extracts (200 mg/kg, IP) in the two phases of the test (Figure 3).
4.3. Hot-Plate Test
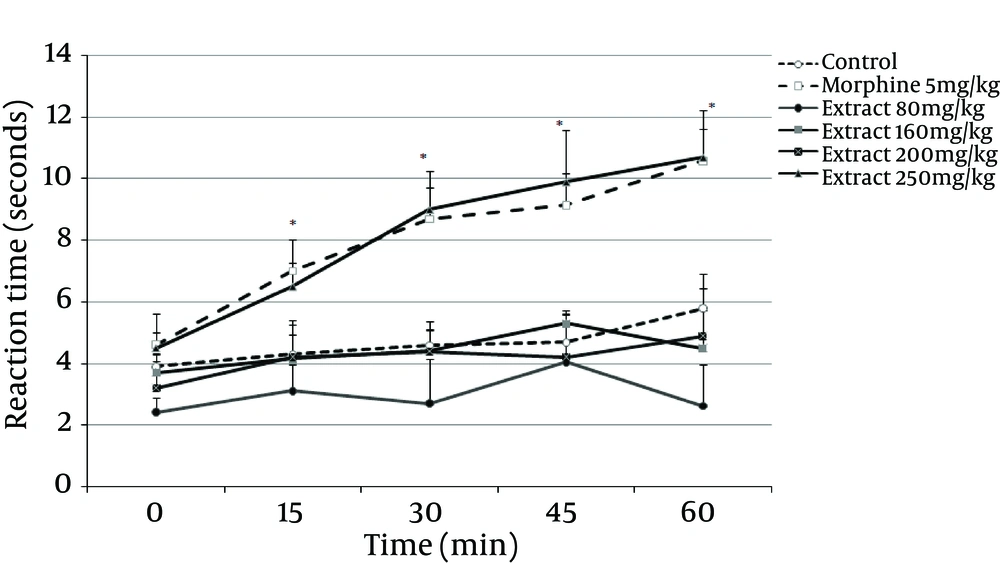
The anti-nociceptive effect of G. vitellinum methanol extracts (80, 160, 200 and 250 mg/kg, IP) was investigated in an acute thermal model of nociception in mice. As evaluated at 15 minutes post-injection, 80, 160 and 200 mg/kg doses of G. vitellinum extract did not produce any significant analgesia, yet the dose of 250 mg/kg reduced acute thermal pain significantly (P = 0.001). The time course assessment demonstrated that the extract began to show anti-nociceptive action in the hot-plate paradigm only 15 minutes after intraperitoneal administration; an effect that lasted for up to one hour post-treatment (Figure 4). Values were found to be significant at 15 (P = 0.001), 30 (P = 0.001), 45 (P = 0.001) and 60 (P = 0.001) minutes after treatment with 250 mg/kg of G. vitellinum extract compared to the saline group. Moreover, similar to morphine, this dose exhibited an anti-nociceptive effect. In the hot plate test, morphine, as the standard drug, produced significant (P = 0.001) analgesia 15 minutes after injection, which lasted for 60 minutes.
Saline, morphine (5 mg/kg; IP) or extract (80, 160, 200 and 250 mg/kg, IP) were administered 15 minutes prior to the placement of the animal on the hot plate and reaction time of mice was measured at 15-minute intervals for one hour. Data represent Mean ± SD of six to eight animals in each group. *P < 0.05 compared with the control group.
5. Discussion
Pain management is probably one of the most common and yet the most difficult aspects in medical practice. Many improved analgesics and anti-inflammatory agents have been developed, but there is considerable opportunity for conceptual innovation. The present study used heat-induced and formalin-induced pain models for evaluating the anti-nociceptive effect of G. vitellinum methanol extract in experimental mice. The formalin test is a model comprised of two distinct phases. The first phase (neurogenic pain) is caused by direct chemical stimulation of nociceptive afferent fibers, predominantly C fibers, which can be suppressed by opiates like morphine (14). The second phase (inflammatory pain) results from the action of inflammatory mediators such as prostaglandins, serotonin and bradykinin in peripheral tissues and from functional changes in the spinal dorsal horn (15). The associated effects, observed using different doses of G. vitellinum methanol extract, showed anti-nociceptive features in both phases, significantly attenuating the pain response similar to morphine. However, it should be noted that pre-treatment with nonselective opioid receptor antagonist (naloxone), reverses the anti-nociceptive effect of methanol extract on formalin-induced pain behavior. Overall, the results strongly suggest that opioid system and central anti-nociception effects were involved in extract-induced anti-nociception. Opioids such as morphine are generally considered to be the most potent and effective antitussive drugs available and are believed to inhibit cough through suppression of a cough center in the central nervous system (16). The results obtained in this study showed that the analgesic effects of G. vitellinum are due to the opioid properties of this plant. The existence of the opioid effect could therefore be responsible for the antitussive effect attributed to this plant in popular medicine. Several plant extracts in ethnomedicine have antitussive effects (17). The mechanism of the antitussive effect of G. vitellinum should be investigated further.
The hot-plate test was used as a thermal nociception model to determine central anti-nociceptive activity. The methanol extract showed analgesic effect implicating both spinal and supraspinal analgesic pathways. Morphine exhibited a rapid effect with a maximum peak of analgesic effect in a short period of time (60 minutes) after administration. Relative to controls, the studied extract significantly increased the mice hot-plat reaction time. The anti-nociceptive activity of the extract at a dose of 250 mg/kg was similar to that of morphine, i.e. starting 15 minutes after the treatment and reaching its maximum analgesic level 60 minutes after administration. Furthermore, in agreement with the results of the first phase of the formalin test, the extract also displayed analgesic effects similar to morphine. Thus, it could be concluded that the methanol extract has anti-nociceptive properties, which is mainly involved in central mechanisms.
Previous studies on G. grandiflorum methanol extract showed significant anti-inflammatory and analgesic activity in the carrageenan, hot-plate and formalin tests (18). Also, another study on the methanol extracts and total alkaloids of G. paucilobum showed graded inhibition of both phases of formalin-induced pain. The extract and alkaloids showed a significant analgesic activity in the hot-plate and writhing tests. Furthermore, the alkaloids were suggested to be the responsible component of the anti-nociceptive and anti-inflammatory actions of G. paucilobum (8).
Cabo et al. reported on the anti-nociceptive properties of G. flavum dried fruit-leaf-root (19). Glaucium flavum contains glaucine, an aporphine alkaloid, which is used as an antitussive. Pinto et al. (12) indicated that glaucine has promising anti-inflammatory, analgesic and antipyretic activity without associated gastric damage.
In summary, this study clearly demonstrated that the methanol extract of G. vitellinum, displayed a potent anti-nociceptive effect in mice at the doses of 200 - 400 mg/kg and acted partly through an opioid-mediated mechanism. Moreover, the anti-nociceptive action demonstrated here supports, at least in part, the ethnomedical uses of this plant. Isolation of pharmacologically active molecules of this plant through directed bioassay is now in progress.