1. Background
Methicillin-resistant Staphylococcus aureus (MRSA) was identified for the first time in the 1960s, showing the response of S. aureus to extensive exposure to penicillins. Increase in MRSA infections seemingly reflects the growing impact of medical interventions, use of various devices, aging (1, 2) and partially is an outcome of advances in patient care and pathogens ability to survive in changing environments (1). The use and probably overuse of antibiotics contribute to the emergence of resistance. Morbidity and mortality rates of MRSA infections in community settings are considerable, just as nosocomial MRSA infections. MRSA now accounts for 160% of S. aureus isolates in intensive care units (ICUs) of the United States hospitals (3) and kills about 19000 hospitalized American patients annually; this is close to the number of deaths because of acquired immune deficiency syndrome (AIDS), viral hepatitis and tuberculosis combined (4, 5). Community-associated MRSA infections were at first identified in children with bloodstream infections who had no prior health-care exposure (6) and growingly reported as a major cause for skin infections and abscesses among healthy adults (7). The incidence of MRSA varies geographically; for example in Europe, a considerable variation exists, with only 0.5% in Iceland but 44% in Greece from 1999 to 2002 (8). There is a constant need for novel antimicrobial substances due to rapid appearance of multiple drug-resistance bacteria (9). Herbal medicines are recognized as a protection system against pathogenic bacteria (10). The genus Juglans (family Juglandaceae) is comprised of several species and is distributed all around the world. Several parts of green walnuts such as its shell, kernel and seed, bark and leaves are used in the pharmaceutical and beauty industries (11, 12). Walnut (Juglans regia Li.) bark is used as a toothbrush and as a dye for coloring the lips in some countries (13). It has been claimed that it owns anti-inflammatory and anticancer properties, helps purifying the blood; it is diuretic and has laxative activities. It contains several therapeutically active constituents, particularly polyphenols (14). Juglans regia stem bark contains chemical constituents, namely β-sitosterol, ascorbic acid5, juglone, folic acid, gallic acid, regiolone and quercetin-3-α-L-arabinoside (9). Antifungal, antibacterial and antioxidant activities of this plant have been studied by several researchers (15, 16).
The extract of Juglans regia bark has shown a wide spectrum of antimicrobial activity in a dose-dependent manner. The acetone extract is the most effective preparation when used as an antimicrobial agent against oral micro-flora (9). This extract has either synergistic or additive effect when tested with a broad spectrum of antibacterial drugs (13). The increasing emphasis on plant studies in the field of medicine is because of antibiotic-resistant bacteria, side effects of chemical antibiotics and their high cost for developing countries. The barks of different species of Juglans regia have been chemically analyzed in numerous studies. Major components in obtained essential oils of Juglans regia include phenolic compounds, terpenoids, alkaloids, flavonoids and steroids (17). It is reported that the bark of Juglans regia Li. contains ketones like juglone, regiolone, sterol and flavonoid (18). Juglans regia bark is a medicinal material used in Iranian folk medicine as an antimicrobial medicine (9).
2. Objectives
The aim of this study was to appraise the antimicrobial activities of hydroalcoholic extract of Juglans regia stem bark against several isolates of MRSA.
3. Materials and Methods
3.1. Collection of Bacteria
From June 2012 to October 2013, a total of 50 strains of S. aureus were isolated from various clinical specimens (urine, abscess, exudates, eyes and joints) from different patients admitted to four medical sciences teaching hospitals of Ahvaz (Golestan, Razi, Aria and Imam Khomeini hospitals). To identify MRSA, swabs were streaked directly onto mannitol salt agar. The agar media were examined after one day (24 hours) of incubation for typical S. aureus colonies. All suspected S. aureus colonies were plated onto blood agar. Identification of S. aureus strains in suspicious colonies was based on measuring production of catalase, DNase test, slide agglutination test (Staphaurex; Murex Biotech Limited) and tube coagulase test. S. aureus strains were identified based on standard tests (19) and S. aureus (PTCC: 33591) was used for quality control of all tests (mecA positive).
3.2. Susceptibility Test of Isolates
Based on disc diffusion method of Kirby-Bauer, one colony of each isolate was transferred into tubes containing 3 mL of brain-heart infusion broth (BHI) and incubated at 37°C for 3 hours to prepare cultures with the standard turbidity (0.5 McFarland standard). In addition, Mueller Hinton (MH) agar was prepared and poured into petri dishes and allowed to cool down and each microorganism was inoculated on the surface of a separate MH agar plate using cotton swabs and several antibiotic discs such as penicillin (10 U), erythromycin (15 μg), ampicillin (10 μg), ciprofloxacin (5 μg), vancomycin (30 μg), clindamycin (2 μg), gentamycin(10 μg) and methicillin (5 μg) (Patan-teb, Iran) were placed on inoculated media surface. After 24 and 48 hours of incubation at 37°C, all plates were read according to the standard procedure (20).
3.3. Polymerase Chain Reaction (PCR) Assay
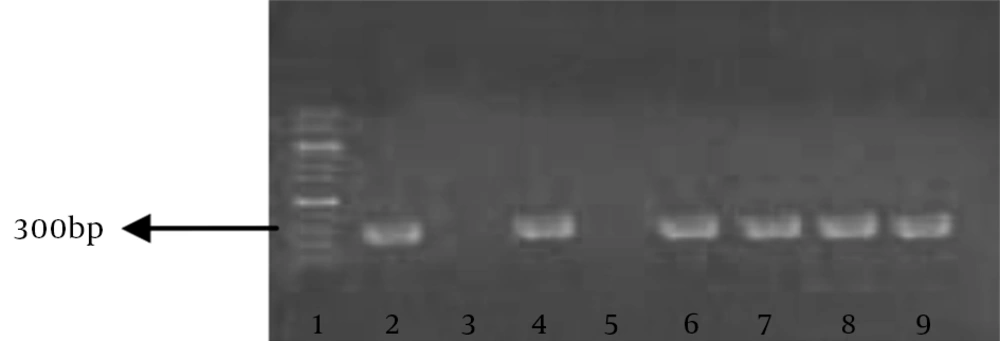
Purified MRSA genomic DNA (the positive control) was prepared from all isolates using a DNA/RNA extraction kit (Viogene, Hungary) and stored at -20°C for PCR. PCR test for detection of MRSA was essentially performed as described by Sambrook and Russell (21) primers (Cinnagen, Iran) used for detection of mecA gene were: mecA1: 5’-TAGAAATGACTGAACGTCCGATAA-3’ and mecA2: 5’-AATTCCACATTGTTTCGGTCTAA-3’ yielding a 307-bp amplicon. PCR cycling conditions were as follows: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of 30 seconds at 94°C, 30 seconds at 50°C and 60 seconds at 72°C, with a final extension step of 10 minutes at 72°C. Ten microliters aliquots loaded onto agarose gel electrophoresis (1.5% agarose, tris-acetic acid- EDTA buffer; 100 V for 30 minutes) and stained with safe stain.
3.4. Preparation of Hydroalcoholic Extract of the Stem Bark
The stem bark of Juglans regia Li. was obtained from a local market. It was ground into fine powder (100 g) and extracted by 500 mL of ethanol 80% (400 mL ethanol 96% + 100 mL water) and kept in room temperature for 48 hours. The extracts were filtered by Whatman filter paper and then concentrated under vacuum conditions at 40°C by means of a rotary evaporator. The obtained residues were stored in a freezer until further tests.
3.5. Determination of Minimum Inhibitory Concentration (MIC)
E-test and broth dilution methods were used to determine MIC. First, a standard turbidity of methicillin resistant S. aureus was prepared in the BHI and inoculated on MH agar (same as before). Then, one gram of hydroalcoholic extract was inoculated in 1 mL of distilled water and made homogeneous (stock: 1000 mg/mL). A serial dilution of extract was prepared in sterile distilled water (500, 250, 125, 62.5, 31.25, 15.62, 7.81 and 3.9 mg/mL). The blank discs (Padtan Teb Co., Iran) were inoculated with 30 μL of every concentration of the extract and placed on a MH agar cultivated with bacterial strains. Negative controls used the same solvents to dissolve the extracts (sterile water) and vancomycin (30 μg) was used as the positive reference. The inoculated plates were incubated at 37°C for 18 hours. The antimicrobial patterns were evaluated by measuring the zone of inhibition against the test bacteria, based on millimeters. The assays were performed in triplicate for each bacterium. Each bacterial sample that showed susceptibility was later tested to determine its MIC.
As for the broth dilution method, 500 µL of 24 hours culture of the test organisms (107 CFU/mL) adjusted to McFarland’s turbidity standard, were incubated in distilled water for 24 hours at 37°C in serial dilutions ranging from 3.9 to 500 mg/mL of the plant extracts. The concentration of the lowest dilution with no detectable bacterial growth was considered as MIC. Absence of growth was confirmed by the absence of turbidity and inoculating into agar (22, 23). To determine the minimum bactericidal concentration (MBC), 0.1 mL of the culture medium was aspirated from each tube of the broth assay that was showing no apparent growth and it was sub-cultured in fresh MHA. After incubation for 24 hours at 37°C, the least concentration with no visible growth on the subculture was considered as the MBC. Each experiment was performed in triplicate.
3.6. Statistical Analysis
Data on zones of inhibition produced by each plant extract and MIC on various bacteria were stored in an excel spreadsheet. Data was analyzed by SPSS v16. Homogeneity of variance test was assessed by Levens test. Average zone of inhibition by each concentration of extract was compared by one-way ANOVA and Dunnett’s post hoc. P value less than 0.05 was considered as statistically significant.
4. Results
Of 50 isolates, all (100%) were found to be methicillin resistant in antibiogram. Nineteen samples (38%) of MRSA isolates were from pus swabs or aspirates and the rest of isolates from other clinical samples. All MRSA isolates were multidrug resistant. In the antibiotic profile of all MRSA strains, very high resistance observed against penicillin, ampicillin and methicillin (100%) and to a lesser degree, erythromycin (48%). Relatively lower degrees of resistance were observed against ciprofloxacin, gentamycin and clindamycin (34%). only one isolate was resistant to vancomycin as shown in Table 1. In the presence of mecA by PCR, 49 (98%) isolates were carrying the resistant gene and 307 bp fragments were observed in electrophoresis (Figure 1). The mean of inhibition zones of hydroalcoholic extract in E-test, negative control (water) and positive control (vancomycin 30 μg/disc) are shown in Table 2. There was a dose-dependent inhibition on tested micro-organisms. The antibacterial activity of this extract at the concentration of 62.5 mg/mL was comparable with that of a standard antibiotic. The results showed that hydroalcoholic extracts had a significant (P < 0.0001) inhibitory effect on the growth of all tested isolates, contrary to the negative control. In the E-test, the size of inhibition zones (25 mm) created by higher concentrations of hydroalcoholic extract (1000, 500 mg/mL) was similar in all isolates. Based on statistical analysis, there were no significant differences between the two lowest (3.9 and 7.81 mg/mL) and two highest (1000 and 500 mg/mL) concentrations (P > 0.05), but all the other concentrations showed significant increase compared to its lower concentration, as shown in Tables 2 and 3. The broth dilution method was used to evaluate antimicrobial activity of hydroalcoholic extract of Juglans regia Li. According to obtained MIC values, hydroalcoholic extract of the stem bark of Juglans regia Li. had the lowest MICs of 15.62 mg/mL on the standard S. aureus strain (PTCC: 33591) and 7.81 mg/mL on MRSA isolates. Minimum bactericidal concentrations of this extract were 15.62 mg/mL and 31.25 mg/mL for S. aureus isolates and the standard strain, respectively.
| Antibiotic | Number of Isolates | ||
|---|---|---|---|
| Susceptible | Moderate | Resistant | |
| Ampicillin | 0 | 0 | 50 |
| Ciprofloxacin | 31 | 2 | 17 |
| Gentamycin | 33 | 0 | 17 |
| Erythromycin | 26 | 0 | 24 |
| Vancomycin | 49 | 0 | 1 |
| Methicillin | 0 | 0 | 50 |
| Clindamycin | 32 | 0 | 18 |
| Penicillin | 0 | 0 | 50 |
| Extract Concentration, mg/mL | Staphylococcus aureus (PTCC: 33591) | Staphylococcus aureus (Isolates) |
|---|---|---|
| 250 | 23 | 23.5 |
| 125 | 22 | 21.5 |
| 62.5 | 18 | 18.5 |
| 31.25 | 16 | 15 |
| 15.62 | 10 | 12 |
| 7.81 | 6 | 0 |
| 3.9 | 0 | 0 |
| Negative control (water) | 0 | 0 |
| Positive control (vancomycin 30 µg) | 17.5 | 17 |
aValues are expressed as mean inhibition zone (mm).
aValues are expressed as mean ± SEM.
bIndicates that the inhibitory zone in the considered concentration is significantly smaller than higher concentration (P < 0.001).
5. Discussion
Methicillin-resistant Staphylococcus aureus (MRSA) is a major reason for nosocomial and community infections. Prevalence of MRSA varies in different countries (24) even between hospitals within a country. It is expected to see more of this problem in places that have better health-care facilities to provide primary care and offer easier access to antibiotics and therefore, receive numerous patients from different regions. Emergence of multidrug resistant MRSA has posed a serious therapeutic challenge, leaving medicinal plants as the drugs of choice. The present study showed that the stem bark of Juglans regia is a potential antimicrobial agent and can be used in surface disinfection. According to our findings, the hydroalcoholic extract indeed had antimicrobial effects against methicillin-resistant S. aureus isolates and the standard strain in low concentrations. In this study, hydroalcoholic extract exhibited zones of inhibition against all MRSA isolates, with the same zones of inhibition against the standard strain with a significant difference in contrast to the control. Also we showed that this effect was dose-dependent. Several studies have been performed to find ways to make use of medicinal plants in the treatment of microbial infections such as in vitro antimicrobial activity screening of some medicinal plants traditionally used against oral pathologic bacteria (9), or in their veterinary applications, for bovine mastitis (25), wounds and gastrointestinal tract complications (26) or as fungistatic (27) and anthelmintic agents (28), all of which have achieved promising results. But until now, no research has been performed on the effects of hydroalcoholic extract of Juglans regia Li. on methicillin-resistant S. aureus. We concluded that the bark of Iranian Juglans regia Li. contains juglone and regionale, which have antibacterial effects against MRSA. The antibacterial properties of the tested plant material may be because of the presence of phenolic compounds, terpenoid, alkaloids, flavonoids and steroids. The bark of Juglans regia Li. contains juglone, regiolane, ketones, sterol and flavonoid (9).