1. Background
Seas and oceans have been recognized as a rich source of metabolites and bioactive compounds with biodiversity and drug activity. Compounds extracted from marine organisms, because of their antifungal and antibacterial activities, have been isolated (1). Sea cucumbers are rich in glycosides (2), especially triterpene glycosides, whose antifungal and antitumor activities have been demonstrated (3). These organisms also have considerable amounts of lectin (4, 5), cerebroside (6), glycosaminoglycan (7, 8), sterol and omega 6, fatty acids and omega 3. The natural compounds in marine organisms can be used as a source of compounds with nutritional, pharmaceutical, and medical applications (9). Compounds with biological activities can be extracted from various animal groups, such as coral, crabs, moss animals, Echinodermata, case-bearers, fishes, and sponges (10).
Echinodermata is an independent and special phylum of animals whose body structure cannot be compared to any other animal. These organisms constitute more than 6,500 species of marine organisms. Sea cucumbers (Holothuroidea) are among the most unusual members of Echinodermata, in terms of both structure and physiology (11). Studies on sea cucumbers have been restricted to its physiology and ecological features for many years (12). Today, sea cucumbers are being tested in terms of their antibacterial, antifungal, anticoagulant, antiviral, cytotoxic, hemolytic, and even anti-HIV activities (13, 14).
Recent studies have shown that sea cucumber extracts have a strong antimicrobial activity. This antimicrobial activity is attributed to triterpene glycosides (15), disulphate glycosides (16), steroid glycosides (17), polyhydroxy sterols (18), naphthoquinone pigments (19), lysozyme, and various precursors (20). Stichopus variegatus (also known as Stichopus hermanni) is a species that is most valued not only as a food source, but it also offers a variety of remedies which have been proven in an animal model system (21). Stichopus variegatus water extract (SVWE) has been reported to be used for rheumatoid arthritis (22), abdominal pain, and liver damage (23), as well as heart ailments (24). Numerous experiments have revealed various in vitro effects, such as antioxidant (25), anti-angiogenesis (26), antimicrobacterial (27), and antileshmanial (28). Experiments have also revealed its potential as a cyctoxic agent on T-lymphoblastic cell lines (29) and its proliferation effect on neurospheres (30).
2. Objectives
In this study, the antimicrobial activity of aqueous methanol, methanol, chloroform, and n-hexane extracts of the sea cucumber (Stichopus variegatus) body wall, collected from Chabahar Bay, in 1 - 8 mg/mL concentrations, were tested in four bacterial species, namely Staphylococcus epidermidis, Proteus vulgaris, Escherichia coli, Staphylococcus aureus, the fungus Aspergillus niger, and a yeast, Candida albicans.
3. Methods
Stichopus variegatus species were collected by divers in the subtidal zone of Chabahar Bay. Samples were placed in a container with sea water and were transferred to the microbiology lab of Chabahar Maritime University, where they were frozen at -20°C. Samples were washed with distilled water after being melted, and their internal organs were removed through an abdominal incision. The dried pieces of the body wall were extracted two times (15). The extraction process was implemented with the aim of separating the solved compounds in a solution or removing compounds from a solid mixture.
There are different techniques, including maceration, digestion, and infusion, that can be employed for extracting the organic compounds from plant and animal tissues. The method for extraction is usually selected in regard to the type of tissue and the extractable material (31). In this study, the maceration technique was used for extracting the body wall of the sea cucumber. The small pieces of the body wall were kept in 300 mL each of aqueous methanol, methanol, chloroform, and n-hexane solutions for 72 hours. After filtering with filter paper, the resulting extracts were condensed under the condition of vacuum with a rotary machine at 40°C and were dried with a freeze drier (15, 31).
Both the antibacterial and antifungal activities of Stichopus variegatus body wall extract, in four concentrations, were analyzed using the disk diffusion method. Initially, several solutions with different concentrations were prepared from the test extracts. Next, they were used for preparing four disks with 1, 2, 4, and 8 mg/mL concentrations. The prepared disks were tested with antibacterial and antifungal experiments. Regarding the studies on other species of sea cucumber, bacterial species Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Proteus volgaris, the yeast Candida albicans, and the fungus Aspergillus niger were used. The McFarland 0.5 standard was used in the preparation of the suspensions of the mentioned microorganisms under a hood. The species were then cultured with a swab on a Mueller-Hinton agar for bacteria and on a Sabouraud dextrose agar for fungi. Subsequently, the disks with the different concentrations were placed on microbiological growth media. Plates containing bacteria were kept in a 37°C incubator for 24 hours, and plates containing fungi were kept at 25°C for 24 - 48 hours. The bacterial and fungal growth inhibitory zones were measured in terms of mm with a caliper (32). In this test, the antibiotics gentamicin and ketoconazole were used as a positive control for bacteria and fungi, respectively.
4. Results
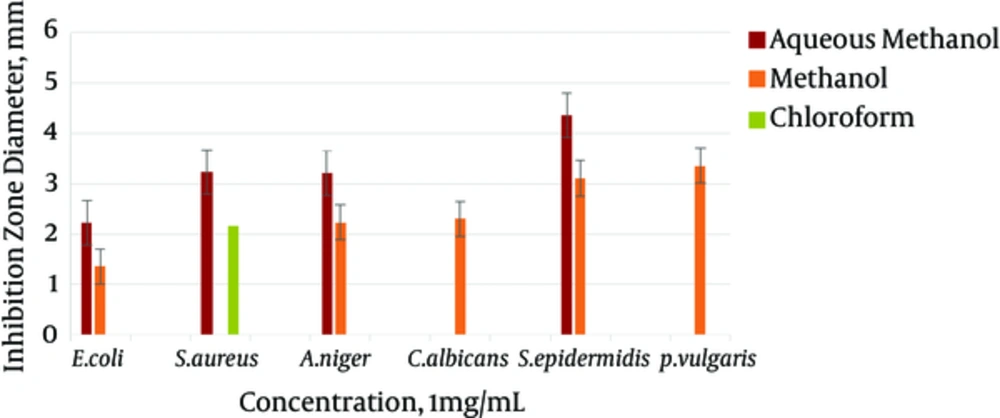
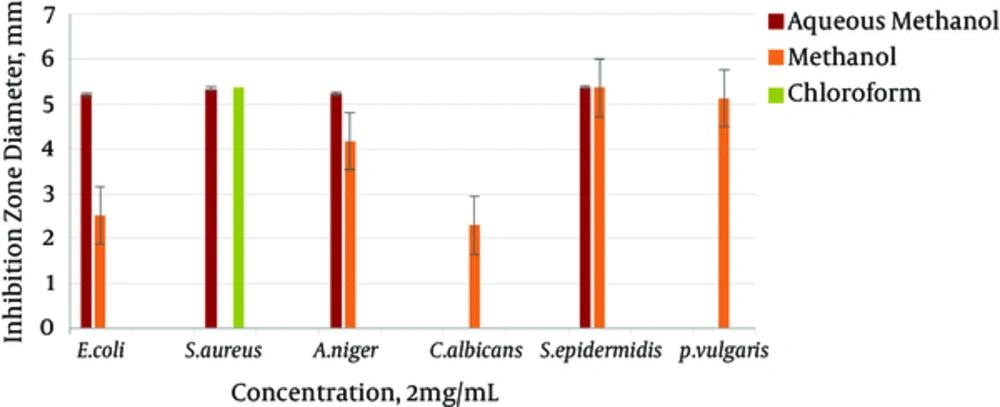
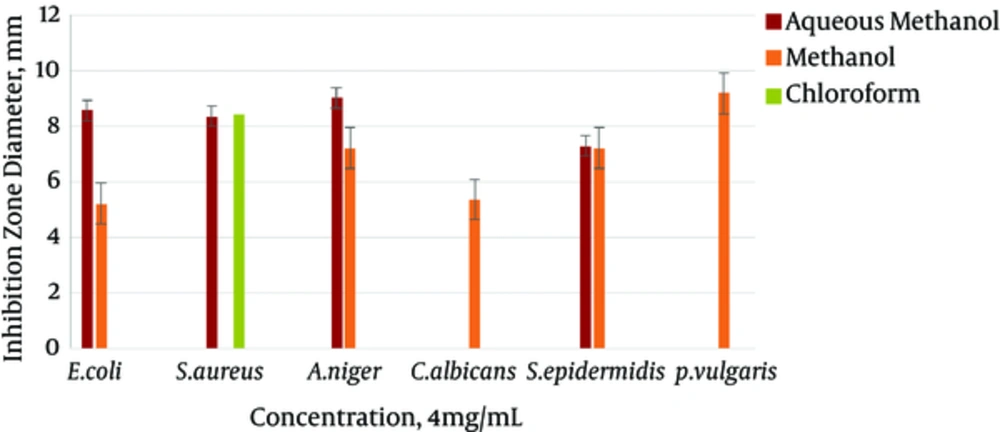
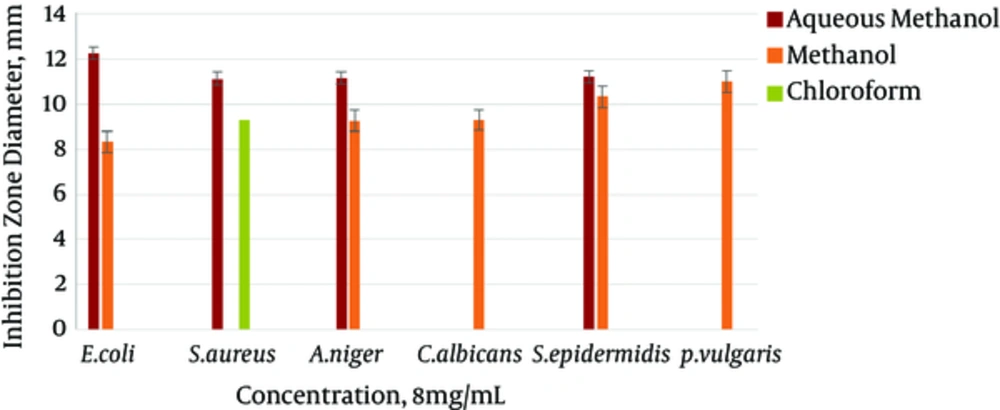
The results gained from testing aqueous methanol, methanol, chloroform, and n-hexane extracts of the body wall of S. variegatus were studied. For the antibacterial and antifungal tests, E. coli, S. epidermidis, and S. aureus were used as bacterial species. C. albicans and A. niger were used as yeast and fungal species. Most chloroform and n-hexane extracts did not show an inhibition zone. The best effect was shown by the aqueous methanol extract of the body wall. It had the highest inhibitory effect on the mentioned strains. The best effect of the aqueous methanol extract, at 8 mg/mL concentration, was related to the E. coli strains, with an inhibition zone of 12.26 mm.
The minimum inhibitory of the methanol extract of the sea cucumber body wall was also on strains of E. coli, at a concentration of 1 mg/mL, with an inhibition zone of 1/36 mm. The maximum inhibitory effect was significant, at a concentration of 8mg/ml of the strain of P. vulgaris, with an inhibition zone of 11 mm. The minimum inhibitory concentration was 1 mg/mL of the E. coli strain, with a 1.36 mm inhibition zone.
The chloroform extract of the sea cucumber body wall affected only the S. aureus strain. Its highest inhibition zone was 9.30 mm, at a concentration of 8 mg/mL, and its lowest inhibition zone was 2.16 mm at a concentration of 1 mg/mL. The n-hexane extract had no effect on the mentioned concentration. The results of the antimicrobial test of white string extracts, at the four concentrations of 1, 2, 4, and 8 mg/mL, are shown in Figures 1 - 4.
5. Discussion
Various countries have organized many studies on the antimicrobial activities of a number of sea organisms, including Echinodermata, during recent years (33). These studies have made it clear that Echinodermata, in contrast to other sea organisms, such as Porifera, Bryozoa, Mollusca, coral, and annelida (i.e., ringed worms), have the highest antimicrobial activity (21). Most experiments carried out to analyze the antibacterial and antifungal activity of sea cucumbers have focused on methanol, aqueous methanol, ethanol, and chloroform extracts, as well as triterpene compounds (34).
Omran and Allam used the disk diffusion method to analyze the antifungal activity of H. polii (a special variety of sea cucumber) in the Mediterranean sea (35). The experiments indicated that the ethanol extract of the body wall of this sea cucumber, in a concentration of 2.5 mg/mL, has a strong antifungal activity on A. Flavus, A. niger, and C. albicans. The antifungal activity of the raw extract (i.e., aqueous methanol) of H. polii, using the disk diffusion method, in the concentration of 150 to 300 μgr/mL, has a strong inhibitory effect on A. funmigatus and a weaker inhibitory effect on Trichophytonrubram, although it did not show any considerable effect on C. albicans.
Comparing this experiment with studies on similar species of C. albicans and A. niger revealed that the aqueous methanol and methanol extracts of the S. variegatus body wall, in the mentioned concentrations, inhibits the growth of C. albicans. By comparing the test with the study on the same strains of A. niger and C. albicans, it can be stated that the methanol and aqueous methanol extracts of the body wall of the sea cucumber S. variegatus, in concentrations of 1 – 8 mg/mL, have an inhibitory effect on A. niger. Only the methanol water extract of S. variegatus, at these concentrations, had an inhibitory effect on the C. albicans strain. Therefore, it can be said that various extracts of different species of sea cucumbers have different effects, even on similar species.
Using the disk diffusion method, Mokhlesi et al. tested the antibacterial and antifungal activities of three extracts (i.e., ethyl-acetate, aqueous methanol, and methanol) of species of H. leucospilota and B. marmorata on S. aureus, E. coli, P. aeruginosa (as bacterial species), A. niger (as a fungal species) and the yeast C. albicans (31). These tests showed that the extracts had no inhibitory effect on the bacterial species (i.e., no inhibition zone was formed). However, A. niger and C. albicans did not grow in the methanol extract of the white strands of H. leucospilota, the methanol extract of the body wall of B. marmorata, or in the aqueous methanol extract of the white strands of B. marmorata. These effects were stronger for A. niger, as the methanol extract of the body wall of B. marmorata, in a concentration of 8 mg/mL, inhibited the growth of A. niger through forming an inhibition zone with a diameter of 17 mm.
However, the results of this experiment showed that the aqueous methanol and methanol extracts of the body wall of S. variegatus, in concentrations of 1 to 8 mg/mL, inhibited the growth of E. coli and A. niger, and the aqueous methanol and chloroform extracts of the body wall of the sea cucumber had an inhibition effect on the growth of S. aureus. The biggest inhibition zone, developed in a concentration of 8 mg/mL for the aqueous methanol extract of E. coli, was 12.26 mm. For the aqueous methanol extract of S. aureus, it was 11.13 mm, and for the aqueous methanol extract of A. niger, it was 11.16, which was attributed to the body wall extract of sea cucumber. As this test indicates, various species of sea cucumbers have different antimicrobial activities. The difference between the molecular mass and amino acid sequence in the extracted peptides from these organisms is what makes their effect on various bacterial and fungal species different.
Another study dealt with the antibacterial and antifungal activities of alcoholic extracts of various species of Holothurian, including A. miliaria, H. scabra, and H. atra on the Tamil Nadu coast, in concentrations varying from 10 to 200 µg/mL. The bacterial and fungal species tested in the study were E. coli, A. hydrophila, Enterococcus Sp., K. pneumonia, P. aeroginosa, S. Typhi, S. aureus, V. harveyi, and Aspergillus sp., which were inhibited by extracts of sea cucumbers including H. atra, H. scarb, and A. miliaris. For example, the raw extract (aqueous methanol) of Holothuria atra, in the concentration of 130 μgr/mL, inhibited the growth of Aeromonas hydrophila by forming a 2 mm inhibition zone, but it did not affect the growth of E. coli. Likewise, the raw extract of Actinopyga miliaris, in the concentration of 200 μg/mL, inhibited the growth of Aspergillus sp. though forming a 4.5 mm inhibition zone. This test convinces us to conclude that, although all of the tested species were limited to a certain geographical location, different inhibition zones were formed by various concentrations. However, our study indicated that the raw extract (aqueous methanol) of the body wall of S. variegatus, in concentrations of 1, 2, 4, and 8 mg/mL, had an inhibitory effect on E. coli and A. niger. Our results make it clear that there are materials with different compounds in the sea cucumber body which contain antimicrobial activity (36).
Comparison of these tests indicates that sea cucumbers have an innate immunity system which can be considered a potential source for discovering antimicrobial peptides. As a result, sea cucumbers can be introduced as a source rich in compounds with antimicrobial features, which means they can be good candidates for synthesizing pharmaceutical and medical compounds and antibiotics.
Patar et al. (2012) studied the effect of the water extract of sea cucumber Stichopus variegatus on rat spinal astrocytes cell lines. The extracts were prepared in four different concentrations of 0.1, 1.0, 5.0, and 10.0 μg/mL. Their results suggest that treatment with S. variegatus water extract induces proliferation and differentiation of spinal astrocytes, in a dose-dependent manner. The potential of S. variegatus water extract as a growth promoter in vitro demands a further analysis of the effects of S. variegatus water extract on spinal astrocytes proliferative in vitro. Some reports show proliferative effects of S. variegatus extract on fibroblast cell lines, neurite growth, blood vessels in vitro, and neural stem/progenitor cells (37).