1. Background
The term “medicinal plants” includes various types of plants used in herbalism. Some of these plants demonstrate medicinal activity. These medicinal plants are considered rich sources of ingredients which can be used in drug development and synthesis.
Herbal medicine has been used for the treatment of various ailments in many countries. Antioxidants, such as vitamin C, polyphones, carotenoids, tocopherols, and flavonoids may be responsible for the antibacterial activity of medicinal plants (1). In addition, these plants play a critical role in the development of human cultures around the world. Some plants are considered important sources of nutrition and, as a result, these plants are recommended for their therapeutic value (2).
Bacterial endophytes are colonizers of the inner plant tissues in which they do not normally cause any substantial morphological changes or disease symptoms. They also are known to enhance the growth and development of host plants. The scientific community has become interested in bioprospecting these microorganisms for their potentially important secondary metabolite production, in particular for application in the pharmaceutical and food industries (3). The question is whether these substances are produced by the plant itself or as a consequence of a mutual relationship with beneficial organisms in their tissue. Much research has shown that in a microbe-plant relationship, endophytes contribute substances that possess various types of bioactivity, such as antibacterial and antifungal (4, 5).
In Iran, extracts from many types of local plants are traditionally used for the treatment of various ailments. In one of our earlier works, regarding antimicrobial activity of endophytes originating from medicinal plants, we examined endophytes from native medicinal plants of Chaharmahal province, in west central Iran, and screened them for antimicrobial activity (6). However, little information is available on the occurrence or the potential significance of bacterial endophytes from medicinal plants Cichorium intybus L, Pelargonium hortorum, and Portulaca oleracea, which seem to be promising medicinal herbs.
2. Objectives
In this descriptive study, we focused on the isolation of bacterial endophytes from three medicinal plants (Cichorium intybus L, Pelargonium hortorum, and Portulaca oleracea) and screened them for activity against the human nosocomial bacterial pathogens Staphylococcus aureus, Acinetobacter baumannii, Enterococcus faecalis, and Pseudomonas aeroginosa.
3. Methods
3.1. Collection of Plant Samples
Samples of three medicinal plants (Cichorium intybus L, Pelargonium hortorum, and Portulaca oleracea) were collected in spring 2013 and, after transferring them to Shahrekord University, they were taxonomically identified at the Botany Department. Asymptomatic leaves and branches of the three medicinal plants were thoroughly washed in running tap water, after which they were surface sterilized by submerging them in 75% ethanol for two minutes. The portions were further sterilized sequentially in 5.3% sodium hypochlorite solution for five minutes and 75% ethanol for 0.5 minutes. After being washed with distilled water and dried, each leaf was divided into segments. For isolation of endophytic bacteria, the disinfected portions were distributed onto the isolation media yeast extract agar (Merck, 64271, Germany) (yeast extract 5gr/L, glucose 10gr/L, agar 16gr/L) (YEA) and peptone agar (15g/L peptone water (Difco, 1807-17-4) and 15g/L agar (Quelab. 42-0223)) (PA) and incubated at room temperature for 3 - 7 days (7). Preliminary bacterial identification was done using Gram staining, catalase and oxidase activity, and biochemical tests on demand.
3.2. Bacterial Strains
Isolates of S. aureus, A. baumannii, E. faecalis, and Ps. aeroginosa were received from Isfahan and Shahrekord hospitals. Early morphological and biochemical tests were done to confirm the genera and species of the received isolates. Biochemical examinations, including Lysine Iron Agar (LIA) (Quelab. 65-2097), Urease (Merck, 8483, Germany), Oxidation-Fermentation (OF), (Merck, 10282, Germany), and Triple Sugar Iron Agar (TSI) (Quelab. 39-4906) tests, in addition to Gram staining, growth in MacConkey agar (Merck, 64271, Germany), and cetrimide media (Biomark, 411-011, India), as well as oxidase (Merck, 3067, Germany) and catalase examinations followed, for confirmation of the isolates. The methods for isolation and identification of all isolates were based on the Quinn et al. guidelines (8).
3.3. Endophytic Bacterial Contents
To isolate the endophytic bacteria, selected colonies were diluted in peptone water (0.1%) and displayed as drops (Pasteur pipette) in PA and YEA media. Petri dishes were incubated at room temperature at 37°C for 24 - 48 hours, simultaneously. The bioassays were conducted using growing colonies in PA and YEA, and the bacteria were inactivated by chloroform for 15 minutes.
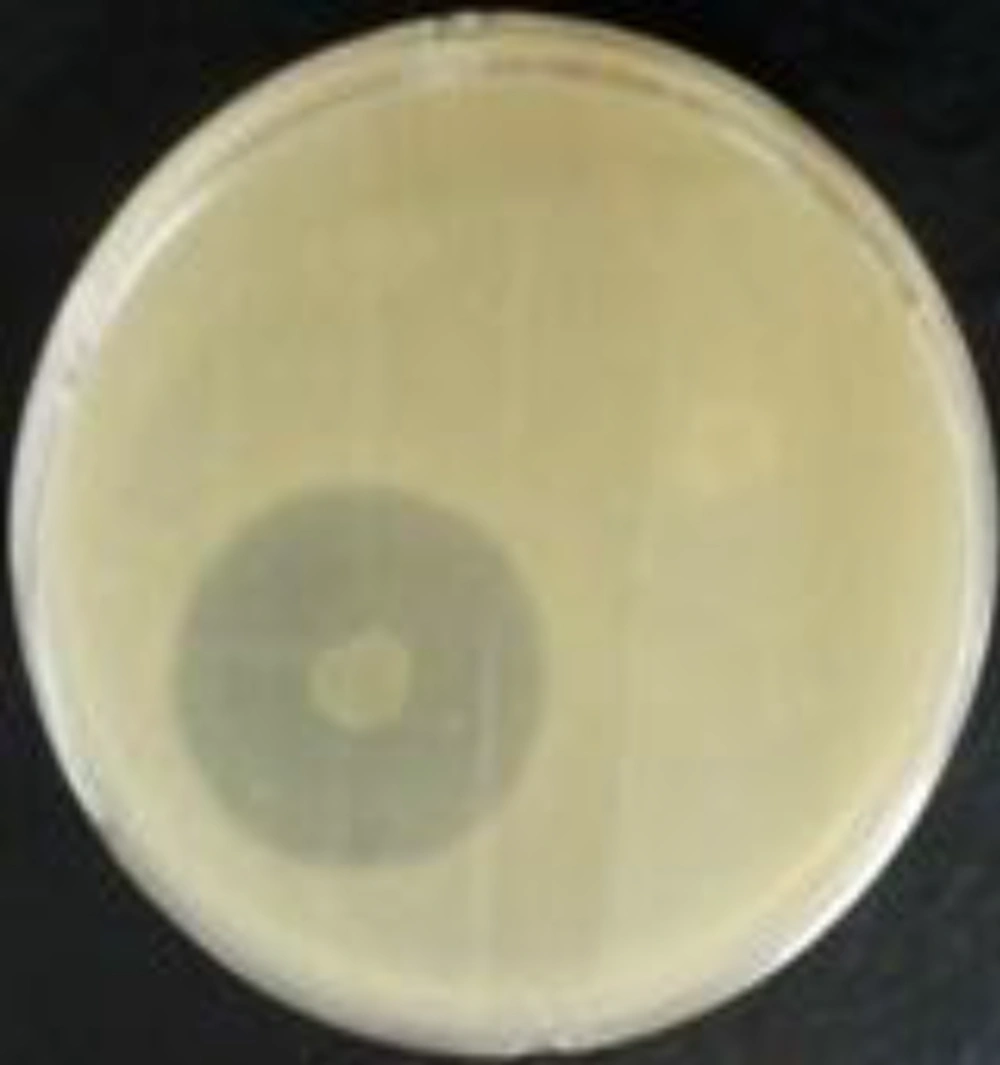
Plates were opened for 30 minutes to evaporate the substance. At the same time, 40 field isolates of S. aureus, A. baumannii, E. faecalis, and Ps. aeroginosa (10 isolates each) (BHI broth 24 hours at 37°C, (Himedia, 400-086)) were reactivated. A volume of 200μL of each culture, properly reactivated, was transferred to 10ml of semisolid BHI medium and shaken. This mixture was deposited onto the surface of plates (YEA) containing chloroform- (Merck, 64271, Germany) inactivated bacterial colonies. The plates were incubated at 37°C for 24 hours for the observation of inhibition halos (7) (Figure 1).
3.4. Endophytic Bacterial Broth Culture
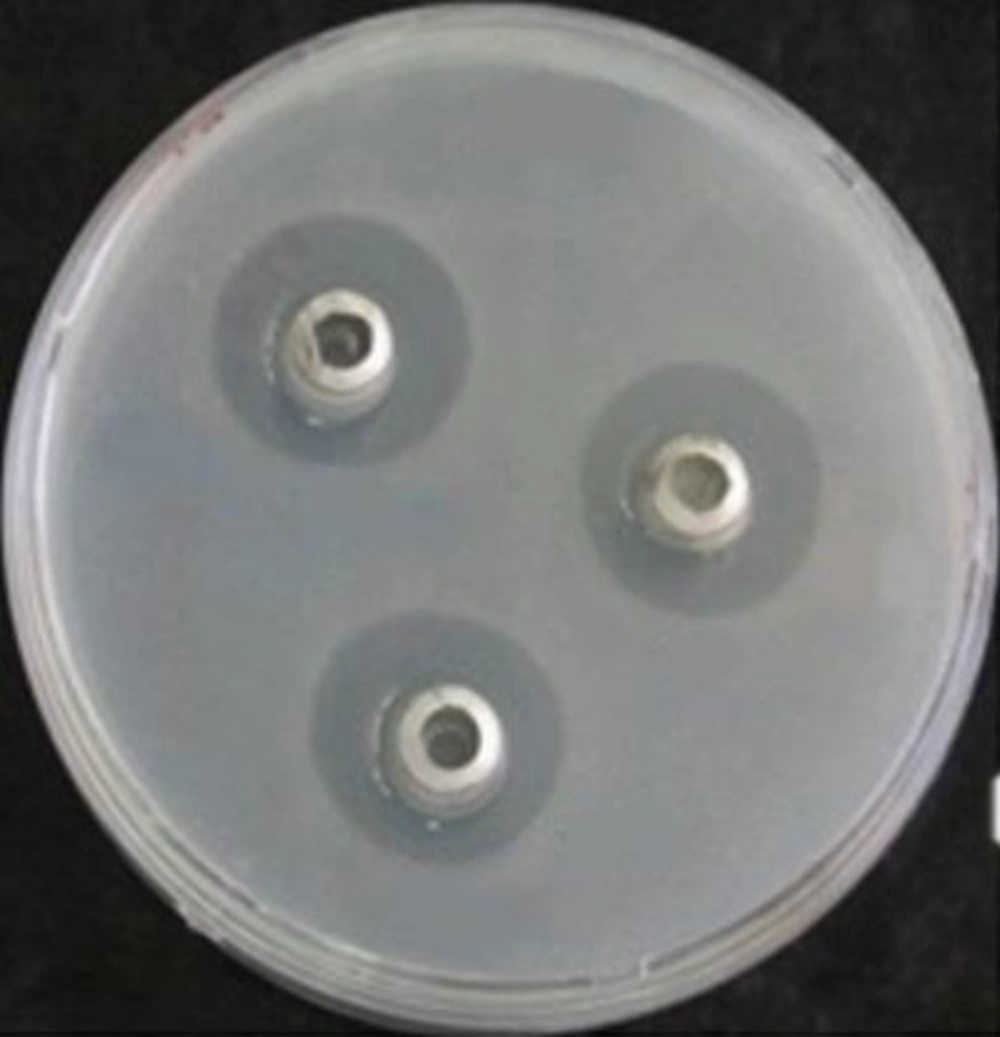
To test the antibacterial activity of the endophytic bacterial culture broth, 200μl of each field isolate (109 CFU mL) was added into 15ml of YEA at 50°C, mixed thoroughly, and poured into a 9 cm-diameter Petri dish. After solidification, two to three sterilized stainless cylinders (5 mm internal diameter and 10mm high) were placed, open end up, on each plate. The culture broth of endophytic bacterial isolates grown in LB broth (Merck, 110285, Germany) (18 - 24 hours incubation at 37°C) was centrifuged (Sigma, serial no. 103286,) at 10,000 rpm for 15 minutes, and filter-sterilized supernatants (100μl of each) were poured into cylinders on each bacterial plate (9) (Figure 2).
4. Results
Segments of surface-sterilized leaves and stems of Cichorium intybusL, Pelargonium hortorum, and Portulaca oleracea incubated in yeast extract agar, peptone water, and brain heart infusion agar plates showed growth of morphologically distinguishable bacterial colonies surrounding the segments after 24 - 48 hours. A total of 24 phenotypically distinguishable bacterial endophytes were isolated in pure form from three medicinal plants. Of the herbs of these 24 isolates, 7 were from Cichorium intybusL (6 leaves, 1 branch), 10 from Pelargonium hortorum (only branch), and 7 were from Portulaca oleracea (3 leaves, 4 branches). The bacterial endophytes were characterized based on micromorphological, Gram staining, and catalase examinations. Of the 24 isolated bacterial endophytes, 5 were Gram-positive (2 cocci and 3 Bacillus spp) and 19 were Gram-negative (7 Bacilli and 12 Coccobacilli). Filamentous forms were not detected in any of the plant samples.
Antimicrobial activity of all bacterial endophytes was assessed against 40 bacterial isolates of S. aureus, A. baumannii, E. faecalis, and Ps. aeroginosa (10 isolates each). The isolate which inhibited growth of any of the test isolate(s) was considered to have antibacterial activity, and the length of the inhibition zone was measured (Tables 1 and 2). Of the 24 isolated endophytes screened, chloroform-inactivated colonies of four endophytes from the leaves and one from the branches of C. intybus L, as well as two endophytes from the leaves and three endophytes from the branches of P. oleracea showed an average inhibition zone of more than 9.5 mm against Staphylococcus aureus and E. faecalis isolates (Table 1), while supernatant culture broth from all 24 endophytes from three plants showed an average inhibition zone of more than 21 mm against S. aureus isolates (Table 2).
| Herb | Endo. | Average Inhibition Zone (mm) | ||||
|---|---|---|---|---|---|---|
| Morph. | E. faecalis | A. baumannii | Ps. aeroginosa | S. aureus | ||
| C. intybus L | 1L | Bacillus spp | 6.8 (2.53) | 0 (0) | 2 (1.8) | 0 (0) |
| 2L | G- Bacilli | 19.4 (5.45) | 0 (0) | 1.5 (1.4) | 10.6 (3) | |
| 3L | Bacillus spp | 23 (5.46) | 0 (0) | 5.3 (3.5) | 14.9 (4.2) | |
| 4L | G+ Cocci | 12.4 (4.01) | 0 (0) | 4.6 (2.25) | 0 (0) | |
| 5L | G- Bacilli | 22.9 (4.54) | 0 (0) | 3.2 (2.03) | 6.9 (2.27) | |
| 6L | G- Bacilli | 5.4 (3.75) | 0 (0) | 3.7 (2.3) | 0 (0) | |
| 1B | G- Cocco bacill | 6.2 (3.48) | 0 (0) | 1.6 (1.5) | 9.8 (3.8) | |
| P. oleracea | 1L | G- Cocco bacill | 2 (0) | 0 (0) | 1.6 (1.5) | 0 (0) |
| 2L | G- Cocco bacill | 12 (1.9) | 0 (0) | 1.5 (1.4) | 0 (0) | |
| 3L | G- Cocco bacill | 11 (1) | 0 (0) | 0 (0) | 0 (0) | |
| 1B | G- Cocco bacill | 0 (0) | 0 (0) | 2.5 (2.3) | 1.2 (1.13) | |
| 2B | G- Cocco bacill | 17.6 (6.03) | (00) | 1.7 (1.6) | 9.8 (3.8) | |
| 3B | G- Cocco bacill | 19.6 (5.4) | 0 (0) | 3.9 (2.4) | 2.8 (1) | |
| 4B | Bacillus spp | 14.6 (4.97) | 0 (0) | 2.7 (2.5) | 6.8 (2.3) | |
| P. hortorum | 1B | G- Bacilli | 13 (4.3) | 0 (0) | 7 (3.5) | 1 (0) |
| 2B | G- Cocco bacill | 20.8 (4.85) | 0 (0) | 1.7 (1.6) | 10 (2) | |
| 3B | G- Bacilli | 3 (0) | 1.5 (0) | 1.5 (0) | 17.8 (3.7) | |
| 4B | G- Bacilli | 4.7 (2.3) | 0 (0) | 1.2 (1.3) | 1.5 (1) | |
| 5B | G- Cocco bacill | 2 (1.8) | 0 (0) | 0 (0) | 1.3 (1.2) | |
| 6B | G- Cocco bacill | 0 (0) | 0 (0) | 1 (0) | 1.5 (1) | |
| 7B | G- Bacilli | 0 (0) | 0 (0) | 3.2 (2.03) | 0 (0) | |
| 8B | G+ Cocci | 1.2 (1.1) | 0 (0) | 0 (0) | 0 (0) | |
| 9B | G- Cocco bacill | 16 (4.47) | 0 (0) | 1.2 (1.13) | 8 (3) | |
| 10B | G- Cocco bacill | 17.2 (4.7) | 0 (0) | 1 (09) | 1.5 (1) | |
aEndo stands for endophytes, morph for morphology, L for leaf, and B for branch.
bNumbers in parentheses show standard deviation of averages.
| Herb | Endo. | Average inhibition zone (mm) | ||||
|---|---|---|---|---|---|---|
| Morph. | E. faecalis | A. baumannii | Ps. aeroginosa | S. aureus | ||
| C. intybus L | 1L | Bacillus spp | 0 (0) | 0 (0) | 2 (1.2) | 21.3 (0.5) |
| 2L | G- Bacilli | 5.4 (2.3) | 3.6 (1. 8) | 1.9 (1.2) | 21.3 (0.6) | |
| 3L | Bacillus spp | 6 (2.3) | 2 (1.2) | 0 (0) | 22.1 (0.96) | |
| 4L | G+ Cocci | 9.8 (2.1) | 1.7 (1.09) | 3 (1.5) | 21.8 (0.9) | |
| 5L | G- Bacilli | 10.3 (2.2) | 2.2 (1.3) | 1.9 (1.2) | 21.7 (0.67) | |
| 6L | G- Bacilli | 10.4 (2.2) | 2.9 (1.8) | 2.2 (1.4) | 22 (0.9) | |
| 1B | G- Cocco bacill | 10.4 (2.2) | 2 (1.2) | 1.9 (1.2) | 21.4 (0.63) | |
| P. oleracea | 1L | G- Cocco bacill | 11 (2.5) | 1.8 (1.1) | 1.5 (1) | 22.5 (0.9) |
| 2L | G- Cocco bacill | 11.9 (2.04) | 2.3 (1.5) | 2 (1) | 22 (1) | |
| 3L | G- Cocco bacill | 10.8 (2.3) | 0 (0) | 2 (1.2) | 21.4 (0.9) | |
| 1B | G- Cocco bacill | 9.9 (2.19) | 2.3 (1.5) | 2.2 (1.4) | 21.3 (0.6) | |
| 2B | G- Cocco bacill | 10.5 (2) | 1.9 (1.2) | 1.7 (1.09) | 21 (0.9) | |
| 3B | G- Cocco bacill | 9.2 (2.03) | 2.1 (1.3) | 2.3 (1.4) | 21.6 (0.9) | |
| 4B | Bacillus spp | 10.9 (2.2) | 1.9 (1.2) | 2.3 (1.4) | 21.2 (0.7) | |
| P. hortorum | 1B | G- Bacilli | 6.4 (2.5) | 3.6 (1.7) | 1.6 (1.5) | 20.9 (1.03) |
| 2B | G- Cocco bacill | 8.2 (2.6) | 1.9 (1.2) | 2.1 (1.4) | 21.6 (1.7) | |
| 3B | G- Bacilli | 8 (2) | 2.1 (1.3) | 1 (0) | 21.5 (1) | |
| 4B | G- Bacilli | 7.8 (2.1) | 1.9 (1.2) | 0.8 (0.7) | 21.2 (1.1) | |
| 5B | G- Cocco bacill | 8.2 (2.3) | 1.5 (1.4) | 2.4 (1.5) | 21 (0.8) | |
| 6B | G- Cocco bacill | 0 (0) | 2.3 (1.5) | 1.7 (1.09) | 21.2 (0.7) | |
| 7B | G- Bacilli | 5.7 (2.2) | 2.2 (1.4) | 2.5 (1.6) | 20.5 (0.7) | |
| 8B | G+ Cocci | 0.8 (0.7) | 1.7 (1.09) | 2 (1.2) | 21.9 (0.88) | |
| 9B | G- Cocco bacill | 5 (2) | 2 (1.2) | 1.8 (1.1) | 21.6 (0.55) | |
| 10B | G- Cocco bacill | 1.4 (1.3) | 1.9 (1.2) | 1.8 (1.1) | 20.9 (0.64) | |
aEndo stands for endophytes, morph for morphology, L for leaf, and B for branch.
bNumbers in parentheses show standard deviation of averages.
Chloroform-inactivated colonies of four endophytes from the leaves of C. intybus L, of two endophytes from the leaves of P. oleracea, and five from the branches of Pe. hortorum showed an average inhibition zone of more than 10.5 mm against S. aureus and E. faecalis isolates (Table 1), while the supernatant culture broth of three endophytes from the leaves and one from the branches of C. intybus L, as well as three from the leaves and four from the branches of Po. oleracea showed an average inhibition zone of more than 9.5mm against E. faecalis isolates. Chloroform-inactivated colonies of five endophytes of Po. oleracea showed an average inhibition zone of more than 10.5mm against E. faecalis isolates (Table 1), while the supernatant culture broth of 24 isolated endophytes of three herbs all showed inhibition zones of less than 4mm against Pseudomonas aeroginosa and A. baumannii isolates (Table 2). The chloroform-inactivated culture broth of 24 isolated endophytes of three herbs all showed inhibition zones of less than 1.5 mm against A. baumannii isolates (Table 1).
5. Discussion
We screened only the stem and leaves of Cichorium intybus L, Pelargonium hortorum, and Portulaca oleracea, although endophytes could also occur in the roots, flower, and seeds. The leaves of C. intybus L were found to harbor more endophytes than the branch segments (Table 1), while for P. hortorum, the condition appeared to be the reverse. This higher species richness in one anatomical site may be attributed to micro-environmental peculiarities, as specific conditions in essential nutrients drive the survival of tissue-specific endophytes. Differences in the prevalence of endophytes in different parts of plants have also been reported by others (10, 11).
Antimicrobial activity of endophytic bacteria is not uncommon. Li et al. (12) have explored endophytic actinomycetes associated with pharmaceutical plants in the rain forest of Yunnan, China and detected endophytic Streptomyces displaying antimicrobial activity against S. aureus, Ps. aeroginosa, and Candida albicans. In one of our earlier works, we showed that most fungal and bacterial endophytes from four medicinal plants (Stachys lavandulifolia, Rumex pulcher, Hypericum scabrum, Starja bachteriarica, and Achillea kellalensis) displayed considerable activity against indicator fungal and bacterial strains (6).
In the present study, five bacterial endophytes from seven endophytes of C. intybus L that were chloroform-inactivated showed antibacterial activity (more than 9.5 mm inhibition zone) against E. faecalis and S. aureus isolates (Table 1). In the supernatant broth culture of bacterial endophytes of this herb, all endophytes from the leaves and branches showed antibacterial activity against S. aureus, and four endophytes showed antibacterial activity against E. faecalis. In each part, one bacterial endophyte showed broad spectrum antimicrobial activity, indicating possible biotechnological applications of endophytes living in the tissues of this herb. However, isolation, purification, and detection of active compound(s) are necessary for their further utilization.
Regarding Pe. hortorum, the supernatant culture broth of all isolated endophytes showed high antibacterial activity against S. aureus (Table 2), but in part of the chloroform-inactivated colonies, five out of ten endophytes of this herb were effective against E. faecalis (Table 1).
Pelargonium species are rich sources of monoterpenes, sesquiterpenes, coumarins, tannins, phenolic acids, cinnamic acids, flavones, flavonoids, and flavonol derivatives (13). The antimicrobial activity of extracts of Pelargonium and their constituents has also been reported by others against S. aureus and some other bacteria (14).
For Po. oleracea, the supernatant culture broth of all isolated endophytes showed high antibacterial activity against S. aureus and E. faecalis (Table 2), but in part of the chloroform-inactivated colonies, only five of seven isolated endophytes were effective against E. faecalis (Table 1). Chan et al. (15) reported that two active ingredients, namely linoleic and oleic acids, were identified from Po. oleracea with synergistic antibacterial activity when combined with erythromycin against MRSA, indicating that they possibly act by inhibiting the efflux pumps of the bacteria cells. The antibacterial activity of the extract of this herb is also reported elsewhere (16). In view of the ever-increasing demand for novel antimicrobial substances, the endophytes identified in examined medicinal plants could be new candidates for a potential source of new antibiotics (3).
In part of the supernatant culture broth, all seven endophytes from C. intybus L showed high antibacterial activity against S. aureus, while four endophytes were effective against E. faecalis (Table 2). In part of the chloroform-inactivated colonies, five endophytes of this herb were effective against E. faecalis and S. aureus (Table 1).
Nandagopal and Kumari reported antibacterial activity of the root extracts of C. intybus L against S. aureus, Ps. aeroginosa, and E. coli (17). Patkowska and Konopinski (18) showed antagonistic activity of selected bacteria of the soil environment of the root of C. intybus L towards fungi pathogenic towards this plant. In terms of each isolated endophyte, antibacterial activities varied considerably against all examined pathogenic bacteria, and also between the two methods (chloroform-inactivated and supernatant culture broth) of examination (Tables 1 and 2), suggesting that bacterial growth inhibition is mediated by a variety of antimicrobial metabolites.
In conclusion, endophytic microorganisms residing in Cichorium intybus L, Portulaca oleracea, and Pelargonium hortorum are a very promising source for production of bioactive compounds effective against some human nosocomial bacterial pathogens. Further research should be conducted to classify the endophytes residing in the studied herbs and exploit the substances produced by them.