1. Background
Burn wound especially to the skin, is an injury caused by fire, heat, caustic agents, electricity or radiation (1). It can be classified into epidermal, partial thickness and full thickness (2). Burn injuries are approximately occurred in 1.25 million cases per year in the United States (3). The prevalence of burning in the developing countries is several times more than Europe and North America and the injuries are also more severe (4). Infection due to the new antibiotic-resistant strains of bacteria is the major impediment topical factor in healing process and also prolonged treatment period (5). Improving the methods of wound healing, tissue repairing and decreasing the time of hospitalization offer tremendous opportunities to enhance the quality of life for the burned patients (6). Silver sulfadiazine (SSD) was introduced in 1970s as an antibacterial agent for topical treatment of burns and wounds (7). SSD cream has broad spectrum antimicrobial activities. It has been shown to inhibit bacteria that are resistant to other antimicrobial agents. Although SSD cream is effective for burns and wounds, but it causes some systemic complications including neutropenia, erythema multiform, crystalluria and methemoglobinemia (8). Although SSD has been considered as the standard treatment for burn wound in the hospital, but alternative therapies which are cheaper and locally available are still practiced (9). The genus Capparis isbelonged to the Capparaceae family. It includes about 250 species, of which several species are distributed in the Mediterranean regions. Various parts of caper have been used for pharmaceutical, cosmetic, and nutritional purposes as well as preventing soil erosion and landscaping. In many countries, the flower buds ,fruits, roots, and seeds of the caper have been used in folk medicine as an anti-rheumatic, tonic, expectorant, anti-spasmodic, diuretic and analgesic agents (10, 11). Furthermore, most of the species have some used as food. According to the official food legislation, commercial capers consumed as condiment are the flower buds of Capparis spinosa L (12). It is a beneficent source of glucosinolates (glucocapparin, glucoiberin, sinigrin, glucobrassicin), flavonoids) rutin, kaempferol), phenolic acids, alkaloids, which are known to provide health-improving benefits due to their various biological activities (antioxidant, anticancerogenic, antimicrobial, antimutagenic) (13). Traditional use of herbal products is recommended in many regions of the world. Cappariss spinosa L may be considered as a valuable and affordable wound healing agent.
2. Objectives
Today, there is a growing interest towards the clinical application of natural drugs for burn wounds healing, therefore the present study aimed to compare the potential wound healing effects of the Capparis spinosa L extract with SSD, as standard drug, on type II superficial skin burning in rats.
3. Methods
3.1. Chemicals
All the used chemicals in this study were of analytical grade and obtained from commercial sources. Silver sulfadiazine powder was purchased from Roche chemical company (Germany).
3.2. Extract Preparation
Fresh and ripe fruits, leaves and petals of Capparis spinosa L were collected from Larestan region in the south of Fars province, Iran and identified at the Herbarium of Department of Pharmacognosy, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Fruits, leaves and petals washed thoroughly in water, cut into small pieces using a mixer, and soaked in an 80% aqueous-ethanol solution in a large container for 72hours with occasional shaking (14). The extracts were filtered through 0.45 μm of filter membrane, concentrated in a rotoevaporator and dried with Freeze Dryer. The obtained extract (dry powder) was calculated 12, 10 and 14% for fruits, leaves and petals, respectively.
3.3. DPPH Assay
The percentage of antioxidant activity (AA %) of each substance was assessed by DPPH assay. The measurement of the DPPH radical scavenging activity was performed according to method described by Brand-Williams et al. (15).
The samples were reacted with the stable DPPH radical in an ethanol solution. The reaction mixture consisted of adding 0.5 mL of sample, 3 mL of absolute ethanol and 0.3 mL of DPPH radical solution 0.5 mM in ethanol. When DPPH reacts with an antioxidant compound, which can donate hydrogen, it is reduced. The changes in color (from deep violet to light yellow) were read [(Absorbance) Abs)] at 517 nm after 100 minutes of the reaction using a UV-VIS spectrophotometer (DU 800; Beckman Coulter, Fullerton, CA, USA). The mixture of ethanol (3.3 mL) and sample (0.5 mL) served as blank. The control solution was prepared by mixing ethanol (3.5 mL) and DPPH radical solution (0.3 mL). AA% was determined according to Mensor et al. (16). The most powerful antioxidant extract was used to make cream.
3.4. Preparation of Cappariss spinosa Cream
Creams were prepared by dissolving different amounts of leaves extract of Cappariss spinosa (2.5, 5 and 10 % in eucerin (w/w)) at 70°C. The two phases were mixed together at the same temperature without vortexing to avoid the entrapment of air. After cooling to 30 - 40°C, the cream was homogenized using a silverson homogenizer at 1500 rpm for 40 minutes (17).
3.5. Animals
Ninety male Wistar rats (180-220 g) were obtained from animal house of Ahvaz Jundishapur University of Medical Sciences. The rats were kept in polypropylene cages and fed with standard rat chow and drinking water ad libitum. The animals were maintained at a controlled condition at 20 ± 2°C with a 12 hour light: 12 hour dark cycle. The investigation was performed according to the animal ethics committee guidelines for the use of experimental animals.
3.6. Burn Wound Model
The back of rats was shaved with a sterilized razor blade. The animals then were returned to their cages for 24 hours to reduce swelling due to shaving. A method described by Kaufman et al. was used for burning (18). The rats were initially anesthetized with single intraperitoneal injections of 6 mg/kg xylazine hydrochloride and 85 mg/kg ketamine hydrochloride. The skin of the animals’ back prepared with 10 % antiseptic povidone-iodine solution. Then the second-degree burn was created by a metal cylinder (1.5 cm ID, self-made) immersed in boiling water (100°C) for 3 minutes and maintained on the back of rats for 5 seconds. All animals were resuscitated immediately with lactate Ringer’s solution (2 mL/100 g body weight) intraperitoneally. Finally, each animal was placed in a separate cage. To avoid variations between experiments, the same researcher made all burns.
3.7. Experimental Groups and Treatments
Group 1 (control group): The burned areas in this group remained without any treatment. Each animal in Groups 2-6 Immediately after burning, received eucerin, SSD 1% and three different doses of CSLE cream (2.5, 5 and 10 % in eucerin, w/w), respectively topically on to the surface of burn areas, twice a day for 14 consecutive days.
3.8. Assessment of Wound Healing
The burn wound site was photographed with a digital camera (Sony Cybershot DSC-P72) on the days 1, 4, 9 and 14. Based on the obtained images from the digital camera, the wound area was measured with Image processing software (Digimizer 5.2.2.0, persianGFX.com). The evaluated surface area was then employed to calculate the percentage of wound contraction, tacking the initial size (measured on day one) of the wound as 100% using the following formula (19).
Wound contraction (%) = (initial wound size-specific day wound size) / (initial wound size) × 100
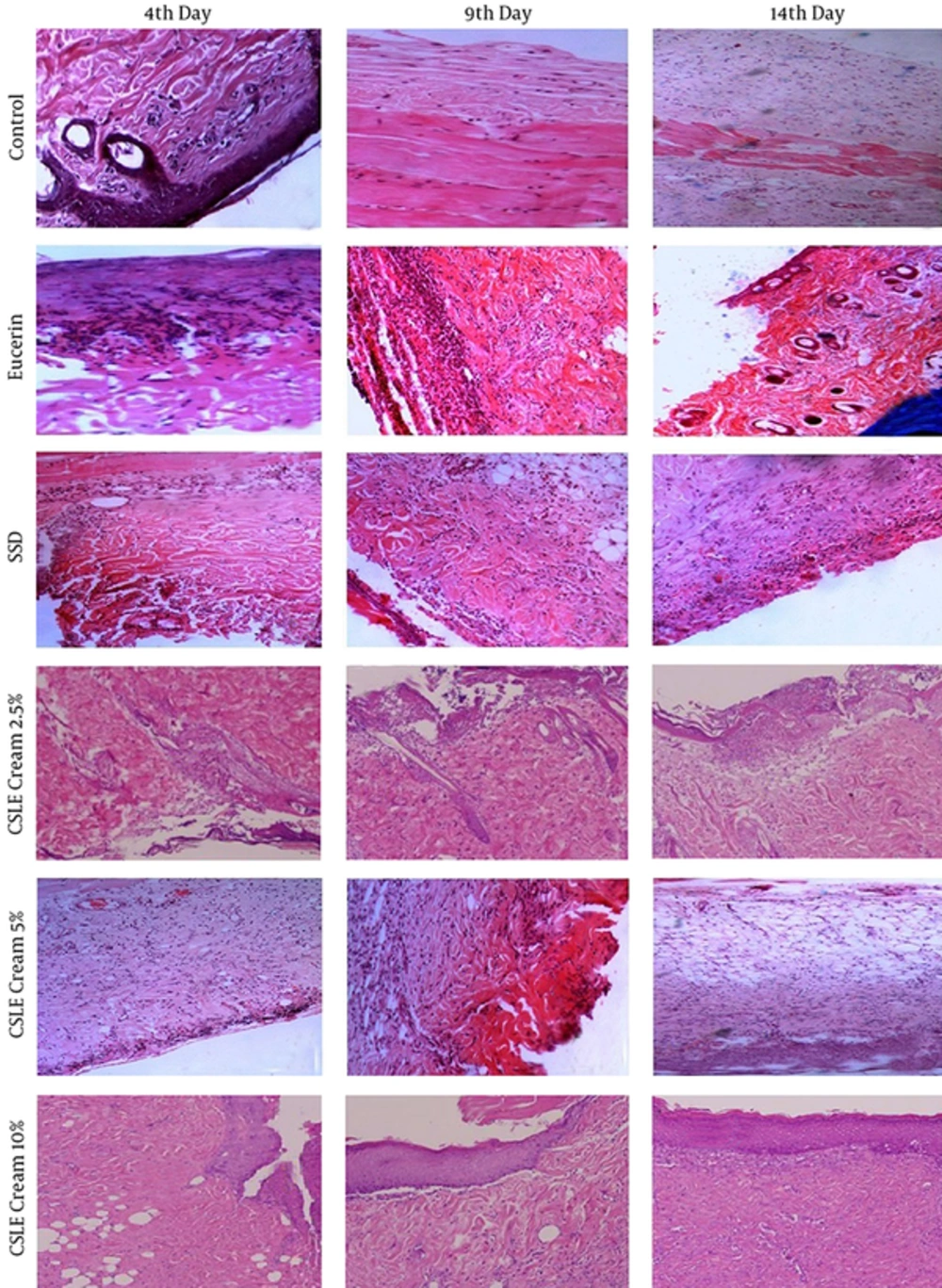
For microscopic studies, 5 rats from each group were scarified on the 4th , 9th and 14th days of experiment and burned area with a little normal skin were sampled. These tissue samples were kept in 10% neutral buffered formalin solution and were sent to the pathologic laboratory of medicine school of Ahvaz Jundishapur University of Medical Sciences. After fixation in formalin solution, the skin tissues embedded in paraffin, cut into 5 μm sections, stained with hematoxilin and eosin dye. The stained slides were observed for re-epithelization, collagen content, inflammatory changes, polymorphonuclear cells, vacuolization and fibroblast. All slides were observed under the light “OLAMPUS” microscope (20).
3.9. Statistical Analysis
The percentage of wound contraction analyzed by the statistical package SPSS 16.0 for Windows. Statistically, all data were expressed as mean ± S.D. Differences between groups mean and between days were estimated using one-way analysis of variance (ANOVA). Statistical significance was set at P < 0.05.
4. Results
The results of antioxidant activities assay showed that the lower IC50 belonged to the leave extract (61.55 µg/mL), and it was 85.76 µg/mL for petals, and 100.19 µg/mL for fruits. The results of the burned areas are shown in Figure 1 and Table 1. Histopathological examination and measurement of the wound area were used as general parameters for the evaluation of wound healing. On day 1, the average diameter of the wound in all groups was almost similar and there was no significant difference between groups (P < 0.05). On the 4th day, the size of wound was decreased in all groups but the percentage of wound contraction in SSD and 2.5% of CSLE groups were significantly greater than other groups (P < 0.05). On the 9th day of the trial, the wound area was significantly lower in all groups compared with the 4th day. Furthermore, wound contraction in SSD and (2.5, 5 and 10%) CSLE groups were significantly greater than control and eucerin groups, but there was no significant difference between SSD group and CSLE cream group. On the 14th day, as shown in table 1, the treated rats with eucerin, SSD, and (2.5, 5 and 10%) of CSLE cream exhibited a significant (P < 0.05) reduction in the size of wound compared with the control group. Additionally, the percentage of wound contraction in the 5 and 10% of CSLE cream group was significantly greater than other groups (Table 1). Moreover there was no significant difference between 5 and 10% of CSLE groups regarding wound-size reduction (P < 0.05).
| Groups | Day | |||
|---|---|---|---|---|
| 1th Day | 4th Day | 9th Day | 14th Day | |
| Control | 16.73 ± 1.33 | 11.74 ± 0.16b | 9.76 ± 0.79b | 6.15 ± 0.39b,c |
| Eucerin | 16.91 ± 0.86 | 12.42 ± 0.96b | 9.99 ± 0.20b | 4.24 ± 0.62b,d |
| SSD | 17.04 ± 1.74 | 10.17 ± 0.98c,d | 7.60 ± 0.52c,d | 3.12 ± 0.34c,d |
| CSLE 2.5 % | 16.69 ± 2.11 | 10.81 ± 0.82b | 8.1 ± 0.45c,d | 3.43 ± 0.46c,d |
| CSLE 5 % | 16.98 ± 1.32 | 11.72 ± 0.73b | 7.24 ± 0.71c,d | 2.52 ± 0.76b,c,d |
| CSLE 10 % | 17.01 ± 1.02 | 11.51 ± 0.67b,c | 7.12 ± 0.63c,d | 2.34 ± 0.39b,c,d |
aEach value represents the mean ± SD (n = 15).
bSignificantly different with the SSD group (P < 0.05).
cSignificantly different with the eucerin group (P < 0.05).
dSignificantly different with the control group (P < 0.05).
Figure 1 shows the histology of control and the treated rats at different days of analysis. Histopatologic observation on the 4th day after injury showed a complete loss of superficialEpithelium, sever inflammation and crust formation in all the experimental groups. In addition, in the 5 and 10%CSLE cream groups, angiogenesis was occurred. On the 9th day, in control and eucerin groups, sever inflammation and mild angiogenesis were observed. In the SSD and CSLE cream groups, acute inflammatory cell infiltration and granulation tissue formation were seen. On the 14th day, collagen deposition was observed in control and eucerin groups (Figure 1). In the SSD group, collagen deposition, chronic inflammatory cell infiltration was observed, and in the CSLE cream-treated groups, collagen deposition and epidermal regeneration were observed. Vascular patterns in the wound bed were similar in SSD and CSLE cream groups. Furthermore, 5 and 10%CSLE cream-treated rats showed a fully re-epithelialization.
5. Discussion
Burn is still a major public health issue all over the word, especially in developing countries. (21). The final aim of the burn management and therapy is wound healing and epithelization as soon as possible (7, 22). Wound healing is a collaborative process involving a variety of cells and matrix components which need to interact continually towards a common goal (23). This process is characterized by three stages; inflammation, proliferation, and remodeling. The proliferative phase typically demonstrates angiogenesis, collagen deposition, granulation tissue formation, epithelialiisation and wound contraction (24). Several studies showed that Natural products with antioxidant properties can accelerate wound healing (25, 26). Furthermore, many researchers reported that C. spinosa has potential antioxidant properties (27, 28). Bonina et al. showed that C. spinosa has photoprotective effects and is able to reduce UVB-induced skin erythema in healthy individuals (28). C. spinosa is a multipurpose plant which contains a number of chemically active and diverse secondary metabolites particularly, flavonoids. Rutin and quercetin are two major flavonoids in caper plant. Moghaddasian et al. developed a HPLC method for simultaneous determination of rutin and quercetin content in different parts of C. spinosa at the floral budding stage.
The highest amount of rutin (25.82 mg/g) and quercetin (10.4 mg/g) was measured in the leaf of caper plant (29). The significant amounts of these antioxidants confirm the nutritional and medicinal value of caper. Ramezani et al. showed that the amounts of rutin in leaves, fruits and flowers of C. spinosa growing wild in Khuzestan, Iran were 61.09, 6.03 and 43.72 mg per 100 g of dried powder, respectively (30).
This finding motivated us to investigated healing effects of C. spinosa on burn wound healing in the experimental animal model. Our finding showed that leave extract of C. spinosa has more potent antioxidant properties compared with other parts of plant, so this extract was used for preparing C. spinosa cream. In current study, there was a significant difference regarding mean percentage of wound contraction between days 4, 9 and 14 of experiment, in all groups. But the most wound contraction was seen in 5 and 10% CSLE cream-treated groups at the final day. The results showed that both C. spinosa cream and SSD significantly (P < 0.05) promoted wound contraction and shortened epithelization period. However, the 5 and 10% cream of C. spinosa seemed to be more effective. These results here are also supported histologically by the increased collagen deposition, angiogenesis, and minimal inflammation in rats treated with 5 and 10% cream of C. spinosa compared with the other groups. Several researchers have been studied the potential effects of natural products on wound healing. In another study, we compared the effect of visceral fat and barley seed ash on burn wound healing in rats. Type II of skin burn was created on the back of the rats. Group one was not treated and considered as control. The burned areas in the second, third and fourth groups were covered twice a day with normal saline, SSD cream and traditional preparation, respectively. The percentage of the burn wound concentration and histopathological examinations were used as the parameters of our study. The results revealed that traditional preparation (visceral fat and barley seed ash) had potential effects on burn wound healing (31). In the study by Yaman et al. healing effects of Nigella sativa and silver sulfadiazine on burn wound in rats was investigated. Histopathological examination was used to evaluate healing effects. They reported that using NS and SSD cream are effective in healing the burned skin wounds in the rat models (32). In another study, Nikzad et al. investigated the effects of Arnebia leaf on the healing process of the second-degree burns. This study shows that Arnebia leaf did not have any effect on healing of the second-degree burn wounds in rats (33). In the study conducted by Shkouhi Sabet Jalali et al., (2007) it has been revealed that using topical honey to treat burns compared with normal saline not only accelerates the healing process, but also has valuable antimicrobial effect to promote the recovery of this kind of wounds (34).
According to the present study, the beneficial effects of 5 and 10% C. spinosa leaves extract cream in the healing of superficial skin wounds in rats is comparable with SSD. Our findings indicated that this herbal product can be applied to treat burns and it can be considered as an alternative therapy.