1. Background
Crocus sativus L., (C. sativus) commonly known as saffron, is widely cultivated in Iran and other countries (1-4). Crocin is a carotenoid isolated from C. sativus and is responsible for the red color of saffron. Crocin is considered as a pharmacologically saffron active component (5). According to several pharmacological studies, crocin can be considered as a new therapeutic agent. Different properties including anticonvulsant (6, 7), antidepressant (8-10), anti-inflammatory and antinociceptive (11, 12), antioxidant and radical scavenging (13), antitumor (14, 15), memory improving (16, 17), and hypotensive (18-20) effects have been attributed to crocin. Crocin also showed protective effects on some toxic agents including diazinon and acrylamide in rat brain, as well as liver and cardiovascular system both in in-vitro and in-vivo experiments (21).
Hypertension is one of the prevalent cardiovascular diseases and is an important risk factor for developing other diseases such as metabolic syndrome, endothelial dysfunction, renal dysfunction, diabetes, congestive heart failure, coronary artery disease and stroke (22). Since some adverse effects have been reported following antihypertensive drugs (23), recently natural plants and their active components have been considered as new antihypertensive drugs with safety, efficacy, cultural acceptability and lesser side effects (24).
The cardiovascular protective effects of saffron and its active components have been established in several studies. It has been shown that the aqueous-ethanol extract of C. sativus possesses a potent inhibitory effect on heart rate and contractility of guinea pig heart via calcium channel-blocking effect (25). Furthermore, the hypotensive effect of C. sativus petals extract has been shown in rats (26). In our previous study, the hypotensive effect of intravenous administration of saffron stigma aqueous extract as well as its two major constitutes, crocin and safranal, in normotensive and hypertensive anaesthetized rats, have been reported (18). In another study, chronic administration of crocin reduced the mean systolic blood pressure in Desoxycorticosterone Acetate (DOCA) salt treated rats (27).
2. Objectives
Considering the hypotensive effect of crocin, the aim of this study was to find whether crocin shows vasodilatory effects on isolated rat aorta. In addition, the mechanism of vasodilatory effects induced by crocin on isolated rat aorta was evaluated.
3. Methods
3.1. Plant
Crocus sativus L. stigma were collected from Ghaen (Khorasan province, northeast Iran) and analyzed in accordance to the ISO/TS 3632-2. Crocin was extracted and purified as defined by Hadizadeh et al. (28).
3.2. Animal
Adult male Wistar rats (weight 200 - 250 g) were provided by the animal center of the school of pharmacy, Mashhad University of Medical Sciences. They were kept on a 12-hour light/dark cycle and at a temperature of 23 ± 1°C with free access to food and water. These conditions were maintained constant throughout the experiments. All animal experiments were carried out in accordance to Mashhad University of Medical Sciences, ethical committee Acts.
3.3. Drugs
Phenylephrine hydrochloride (PE), acetylcholine chloride (ACh), NG-nitro-L-arginine methyl ester (L-NAME) were purchased from Sigma-Aldrich (Germany). Other chemicals used in this study were provided by Merck (Germany).
3.4. Tissue Preparation
Rats were killed by intraperitoneal (IP.) injection of ketamine/xylazine. Then, the thorax was opened and the thoracic aorta was quickly removed and cleaned from adherent connective tissues and cut into rings (4 - 5 mm in length). Special care was taken to ensure that the endothelial was not damaged during tissue preparation. Two stainless steel stirrups were passed through the lumen of each ring. One stirrup was connected to an isometric force transducer (POWERLAB, AD Instrument, Australia) to measure tension in the vessels. The rings were placed in a 25-mL organ chamber (Organ Bath, 4 channels, AD Instrument, Australia) containing Krebs solution gassed with 95% O2/5% CO2, and maintained at 37°C. The composition of Krebs solution was as follows: 118.0 mM NaCl, 4.7 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 25.0 mM NaHCO3, 11.1 mM glucose, and 2.5 mM CaCl2 (pH = 7.4) (29). Rings were placed under a resting tension of 2 g (in preliminary studies determined to be optimum), and allowed to equilibrate for one hour. During this equilibrium period the physiological salt solution was replaced every 15 minutes. The lab chart 7.3 (AD-Instruments) software was used for this study.
3.5. Vasodilatory Effects of Crocin on the Thoracic Aorta Rings
To study the vasodilatory effects of crocin, isolated rat thoracic aorta rings were contracted by 10-6 M PE or KCl 80 mM in two separate experiments, when the vasoconstriction curves of rings reached the plateau phase of the maximum tension, crocin (0.1, 0.2, 0.4 and 0.8 M) was added, and the tensions were recorded. The vasodilatory effect of crocin was expressed as a percentage of relaxation to maximum constriction induced by 10-6 M PE or KCl 80 mM.
The vasodilatory effect of crocin on constriction, induced by PE on rat thoracic aorta, was also performed both on intact endothelium and denuded endothelium rings. The presence of functional endothelium was confirmed by the ability of ACh (10-5 M) to induce ≥ 80% relaxation of rings precontracted with PE (10-6 M).
To examine whether crocin-induced vasorelaxation is mediated by the NO, aortic rings were incubated by L-NAME (10-5 M) for 20 minutes. Then, thoracic aortic rings were contracted by 10-6 M PE and the vasorelaxant effect of crocin (0.1, 0.2, 0.4 and 0.8 M) was then examined.
3.6. Statistical Analysis
All results are expressed as mean ± Standard Error of the Mean (SEM). Analysis of variance (ANOVA) followed by Tukey-Kramer tests were performed to compare means. P values less than 0.05 were considered significant.
4. Results
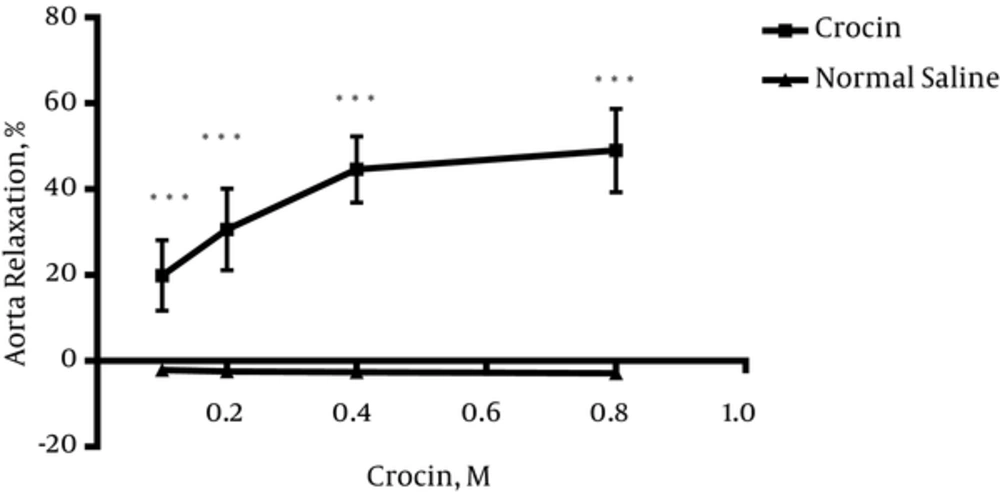
4.1. Crocin-Induced Vasorelaxation in Endothelium-Intact Aortic Rings Precontracted by PE
As shown in Figure 1, crocin (0.1, 0.2, 0.4 and 0.8 M) induced relaxation in endothelium-intact aortic rings precontracted with PE (10-6 M) in a concentration dependent manner. Maximum relaxation response induced by the highest concentration of crocin (0.8 M) was about 50%.
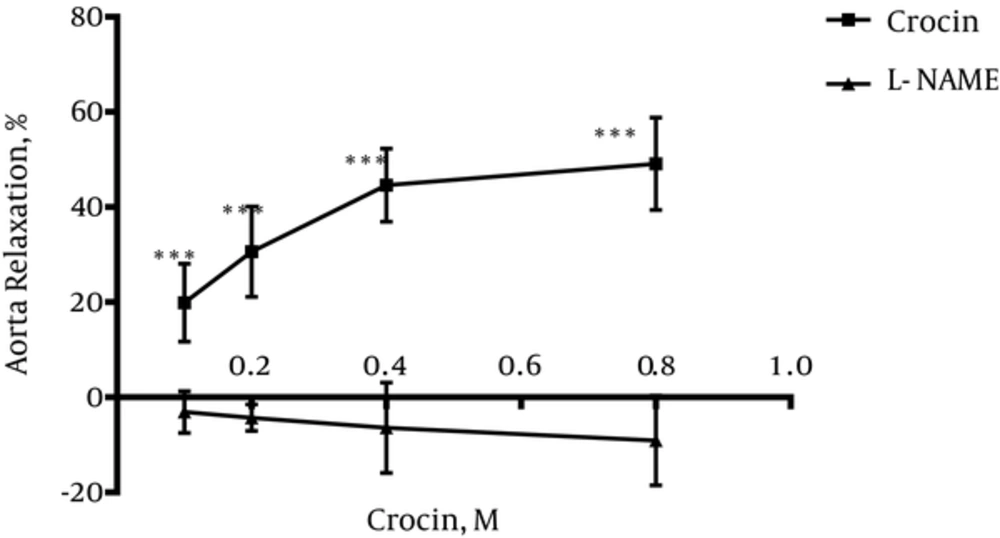
4.2. Vasodilatory Effect of Crocin in Endothelium-Intact Aortic Rings Pre-Incubated With L-NAME
Incubation with L-NAME for 20 minutes led to the significant differences in relaxation induced by crocin at different concentrations in endothelium-intact aortic rings precontracted with PE (10-6 M) in comparison with relaxation induced by crocin in the absence of L-NAME (P < 0.001) (Figure 2).
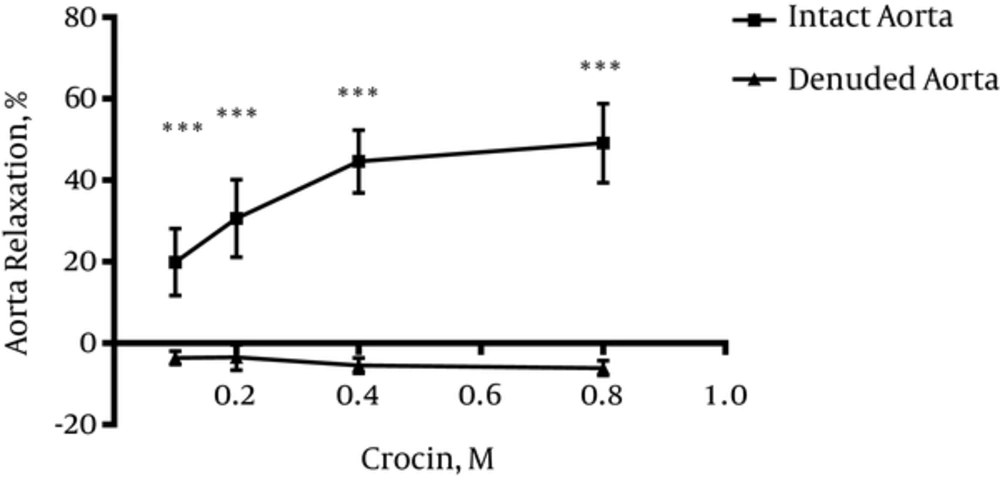
4.3. Vasodilatory Effect of Crocin in Endothelium Denuded Aortic Rings
Significant differences were observed between relaxation induced by crocin (0.1, 0.2, 0.4 and 0.8 M) in endothelium intact and endothelium denuded aortic rings (P < 0.001) (Figure 3).
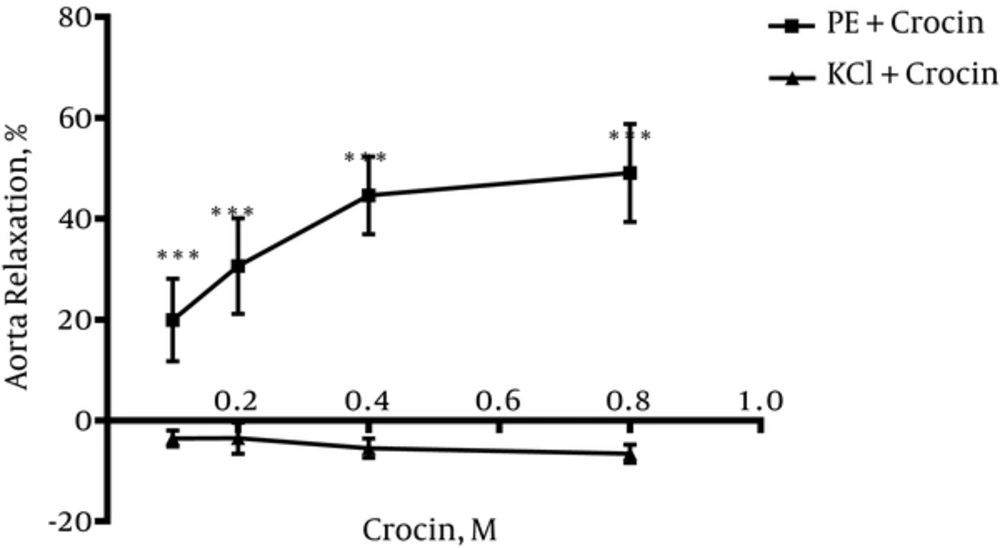
4.4. Crocin Could Not Induce Vasorelaxation in Endothelium-Intact Aortic Rings Precontracted by KCl
As shown in Figure 4, crocin could not induce relaxation in endothelium-intact aortic rings precontracted with KCl (80 mM). Significant differences between relaxation induced by crocin in endothelium-intact aortic rings precontracted by KCl and PE were observed (P < 0.001) (Figure 4).
5. Discussion
This study revealed that crocin could induce vasorelaxation in isolated rat aorta through an endothelium-dependent mechanism.
According to several investigations crocin is able to reduce blood pressure. Intravenous administration of crocin dose-dependently reduced the mean arterial blood pressure in normotensive and hypertensive anaesthetized rats. For example injection of 200 mg/kg crocin induced 51 ± 3.8 mmHg reductions in mean arterial blood pressure. The hypotensive effect of crocin in hypertensive animals was more than normotensives. This hypotensive effect did not induce reflex tachycardia (18). Another study revealed that crocin decreased the mean systolic blood pressure in deoxycorticosterone acetate (DOCA) salt-treated rats in chronic administration (27). As crocin caused reduction in blood pressure, in this study the mechanism of crocin hypotensive effect in isolated rat aortic rings was evaluated.
Based on the results, crocin induced vasorelaxation in endothelium intact aortic rings precontracted with PE. To investigate whether the relaxing effect of crocin depended on endothelium-mediated mechanisms, endothelium derived vasodilator relaxing factor such as nitric oxide was evaluated (30, 31). Nitric oxide is an endothelium releasing factor, which produces by endothelial nitric oxide synthase (eNOS) and induces vascular smooth muscle relaxation through the activation of guanylyl cyclase (32). Nitric oxide has an essential role in the regulation of normal and pathological blood pressure (32).
To evaluate the role of NO in relaxation induced by crocin, the aortic rings were incubated by L-NAME; L-NAME is an inhibitor of nitric oxide synthase. Inhibition of NO by L-NAME inhibited the relaxation induced by crocin. This result showed that NO is involved in crocin relaxant activity.
To verify endothelium-dependent relaxant activity of crocin, another experiment was carried out on endothelium denuded aortic rings. Results indicated that endothelium removal abolished the observed relaxant response in intact endothelium. Therefore, it can be concluded that relaxation effects of crocin is endothelium-dependent. In this study, the effect of crocin on rat isolated aortic rings precontracted by KCl was also evaluated. It is believed that KCl depolarizes the cell membrane and increases intracellular calcium via voltage dependent calcium channels of smooth muscle cells and this process leads to contraction whereas, PE (an a1-receptor agonist) activates phosphoinositide signal transduction, and the induced contraction involves receptor-operated calcium and voltage operated calcium channels (33). Results showed that crocin could not induce relaxation in endothelium-intact aortic rings precontracted by KCl, thus, according to this result, it is suggested that L-type calcium channels might not be affected by crocin.
It was reported that crocetin extracted from Gardenia jasminoides improved endothelium-dependent relaxation of aorta isolated from hypercholesterolemic rabbit. The protection induced by crocetin has been attributed to the increased activity of eNOS, which led to the increase of NO production and finally protection against atherosclerosis. It was also indicated that down regulation of eNOS expression as a result of oxidized low density lipoprotein (LDL) and increased NO production in bovine aortic endothelial cells (BAECs) were inhibited by crocetin (34). Result of another study showed that crocin could improve vascular toxic effects of diazinon by restoring altered contractile responses to PE and KCl and impaired relaxant responses to acetylcholine and sodium nitroprusside in rat aorta (35). Moreover, crocin restored the effects of sub chronic diazinon administration on systolic blood pressure in rats (36). Taken together, crocin could increase NO production and induce relaxant activity in aortic rings isolated from rats, dose dependently.
In summary, the results of the present study indicated that crocin induced relaxation in isolated rat aortic rings might be due mainly to its effect on endothelium via nitric oxide synthase pathway.