1. Background
Blood disorders always created many problems for patients. Regular blood transfusion should be performed to treat some patients with blood disorders; this has some adverse effects such as iron overload and deposition in vital organs such as the heart, liver and endocrine glands of patients. Suppression and reduction of blood leukocytes in patients with immunodeficiency or those undergoing chemotherapy or corticosteroids treatment predispose patients to a variety of diseases and infections. In today's advanced world, a common disorder is anemia, which has several causes, including destruction of membrane of red blood cells due to free radicals and treatment of anemia as a result of using some medicinal plants that are potentially absorbent of free radicals (1). Traditional medicine in developing countries plays an important role in maintaining health of people and plants are a major supply source of drugs (2). Fennel with the scientific name of the Foeniculum vulgare mill is a member of the Apiaceae family (3). F. vulgare has the effects of bracing, stomach tonic, appetizer, emmenagogue, milk secretion-enhancing and carminative (4). Fennel oil inhibits growth of Enterococcus and Clostridium perfringens in the rat colon (5) and has also antifungal effects (6, 7). Estonian extract of fennel has immunomodulatory effects on stimulation of peritoneal macrophages of mice and production of nitric oxide and ROS and being fatal to Candida albicans (8). In addition, fennel has antioxidant activity and its oils has pro-oxidant effects (9, 10). Fennel has protective effects on the liver by decreasing AST, ALT and ALK-P levels (11).
Regarding antimicrobial resistance and adverse effects reported for some chemical medicines, scientific approach to plants and their consumption has been increased in recent years; therefore, scientific research is necessary to determine the pharmacological properties of plants.
2. Objectives
Fennel herb due to antioxidant properties and immunomodulatory effects would probably affect hematological indices, and because no study has been performed in this regard, the aim of this study was to evaluate the effect of hydro-alcoholic extract of fennel on hematological indices in rats.
3. Materials and Methods
3.1. Animals
Thirty male Wistar rats (170-220 grams) were prepared from animal house central of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals were maintained in plastic cages with 12/12 h light/dark cycle at 21 ± 2°C. These conditions were maintained constant throughout the experiments. All the ethical issues were considered based on Ahvaz Medical University Ethical Protocols (AMUEP) in animal experiments.
3.2. Extraction
Fennel seeds were prepared by the department of Pharmacognosy; faculty of Pharmacy of Ahvaz Jundishapur University verified the species as Foeniculum vulgare mill. For preparing the extract by maceration method, initially 100 grams fennel seed was weighed and after milling, it was poured into the Erlenmeyer flask. Then 80% ethanol solution (Merck, Germany) was added four times more than the weight of the plant and maintained for three to four days on the shaker at lab temperature. After this period, the mixture was passed through filter paper and the solvent extracts were evaporated on a watch glass at 25°C Ben Marie. Extract powder was obtained and kept at 4°C until use (12, 13). Concentrations of 250, 500, 750 and 1000 mg/kg/BW were prepared from powder obtaining of fennel using normal saline solvent (Daru Pakhsh, Iran) (14).
3.3. Grouping and Prescribing the Extract
Thirty rats were divided into six groups of five rats as control, and sham groups and four experimental groups of 1, 2, 3 and 4. The control group did not receive any treatment, the sham group received 1 mL normal saline ( as an extraction solvent) and the experimental groups 1, 2, 3 and 4 respectively received 1 mL hydro-alcoholic extract of fennel in four doses of 250, 500, 750 and 1000 mg/kg/BW every 48 hours for 30 days by gavage (15).
3.4. Experimental Tests
One day after the last gavage, rats were anesthetized with ketamine 10% (60 mg/kg) and xylazine 5% (10 mg/kg) (Alfasan-Holland). For counting RBC, 2 mL of blood was taken from the heart of rats and EDTA (Ethylene-Diamine-Tetra-Acetic acid) was added to prevent it from coagulation. Red blood cell was counted using a diluent solution of Heim A and a hemocytometer slide by light microscope (Olympus, 3H-Z-Japan). For counting WBC, diluent solution of Marcanu and hemocytometer slide was used. Hematocrit (HCT) was calculated using a small capillary tube and centrifuged (Hawksley, England) (16), Then, hemoglobin (Hb) was measured using a colorimetric assay via Drabkin's diluent solution. For calculating the coagulation time, about 5 mm was cut from the bottom of the tail and a drop of blood was laid on the slide and coagulation time was calculated by a drop method on the slide. Bleeding time was also measured by placing the tail in normal saline solution at 37°C and continued blood flow until cessation of and blood flow (17).
3.5. Statistical Analysis
SPSS 15 software (IBM, US), ANOVA and LSD backup test were used to determine differences between the experimental and control groups. Mean ± SD was considered significant if P < 0.05.
4. Results
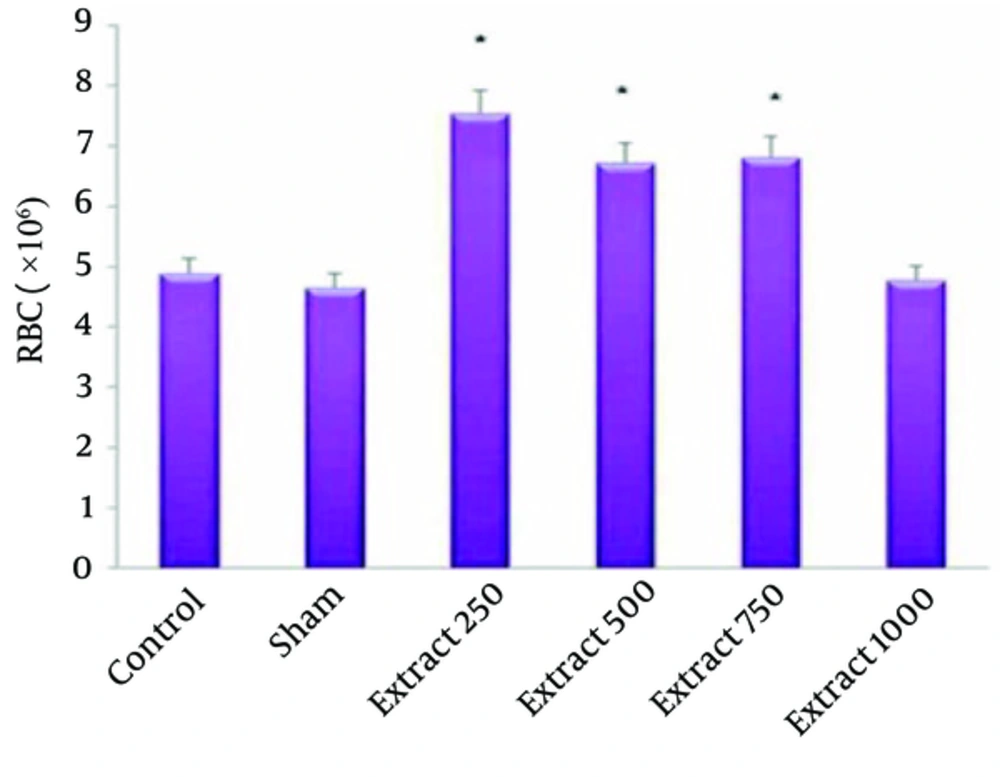
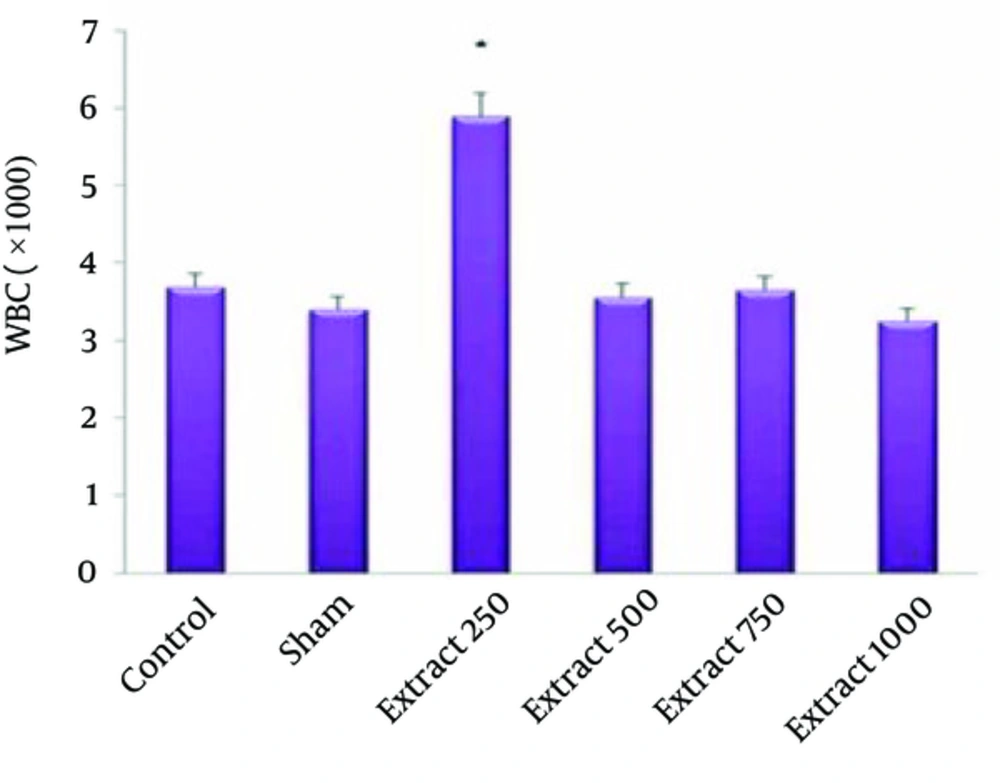
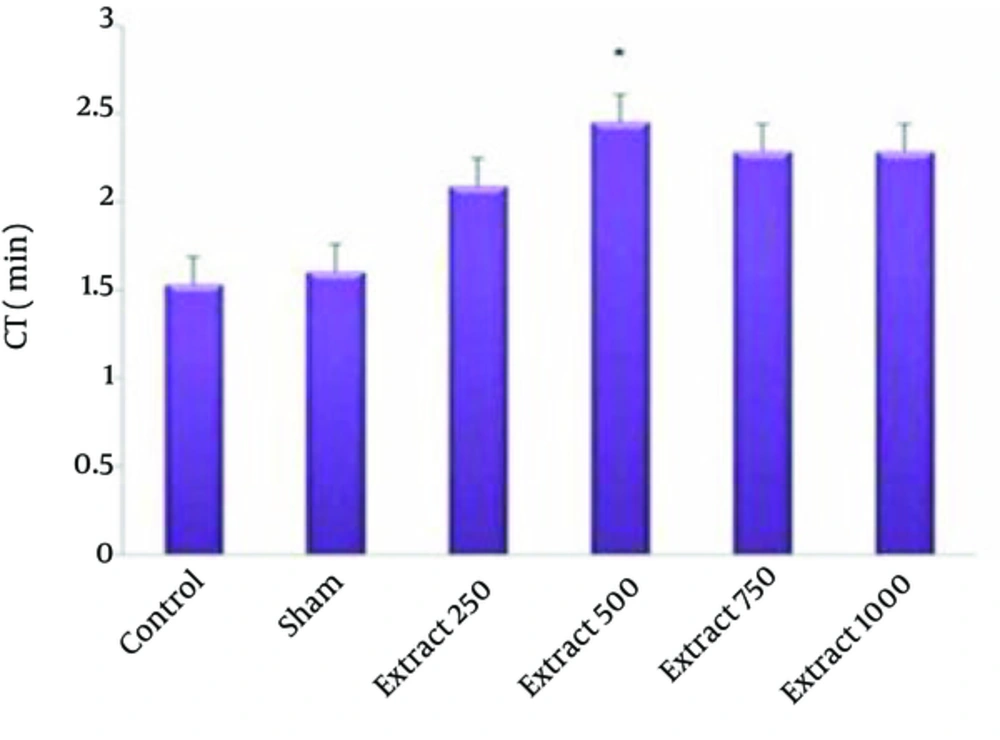
In Table 1 we can compare the Mean ± SE of hematological indices examined in this study. The effect of hydro-alcoholic extract of fennel on red blood cells, white blood cells and blood coagulation time is shown in Figures 1-3.
| Groups | RBC × 100000/ µL | WBC × 1000/µL | HCT, % | Hb, g/dL | CT, min | BT, min |
|---|---|---|---|---|---|---|
| Experimental 1 | 7.54 ± 0.54 d | 5.89 ± 0.78 d | 43.20 ± 1.45 | 11.49 ± 0.37 | 2.09 ± 0.09 | 3.07 ± 0.99 |
| Experimental 2 | 6.72 ± 0.19 d | 2.13 ± 0.39 | 42.60 ± 1.40 | 12.06 ± 0.56 | 2.45 ± 0.2 d | 3.02 ± 1.59 |
| Experimental 3 | 6.82 ± 0.72 d | 3.65 ± 0.72 | 40.00 ± 1.09 | 10.00 ± 0.49 | 2.28 ± 0.15 | 3.00 ± 0.50 |
| Experimental 4 | 4.77 ± 0.25 | 2.12 ± 0.56 | 41.60 ± 1.16 | 12.34 ± 0.53 | 2.28 ± 0.2 | 3.58 ± 1.90 |
| Control | 4.89 ± 1.18 | 3.68 ± 0.77 | 43.80 ± 0.80 | 11.37 ± 0.67 | 1.53 ± 0.05 | 0.40 3.41 ± |
| Sham | 4.65 ± 0. 85 | 2.02 ± 0.30 | 41.80 ± 1.52 | 11.49 ± 0.36 | 1.6 ± 0.06 | 3.14 ± 0.79 |
a Groups 1, 2, 3 and 4 received respectively doses of 250, 500, 750 and 1000 (mg/kg/BW) of F.vulgare extract and sham received normal saline.
b Abbreviations: RBC, Red blood cell; WBC, White Blood Cell; HCT, Hematocrit; Hb, Hemoglobin; CT, Clotting Time; BT, Bleeding Time.
c Data are presented as Mean ± SE.
d P < 0.05 indicating significant differences between experimental and control groups.
The mean RBC count in the experimental groups of 1, 2 and 3 compared to the control groups showed a significant increase (P < 0.05) (Figure 1). The mean WBC count of experimental group 1 compared with the control group showed a significant increase (P < 0.05) (Figure 2). In the experimental group 2, increased coagulation time compared to control group was observed (Figure 3). The study of other hematologic indices showed no significant changes compared to the control group (P > 0.05).
5. Discussion
Although medicinal plants show less therapeutic effects compared to synthetic drugs, they have more acceptance than chemical drugs in various communities, because their adverse effects are less common. In recent years, plenty of studies conducted on the fennel plant. The results of this study showed that fennel extract increased the number of RBCs in healthy rats significantly (Table 1, Figure 1). By their antioxidant properties, plants can neutralize adverse effects of free radicals. New studies have shown that free radicals cause the destruction of cell membrane of RBC. Moreover, by increasing the stability of membrane cells due to their antioxidant properties, medicinal plants can be effective in the treatment of anemia. By its antioxidant effect Madagascariensis harungana can protect the membrane of red blood cell and its membranous process and be useful in the treatment of anemia (1). Dehghani et al. studied the effect of hydro-alcoholic extract of the Cirsium vulgare plant on blood cells in rats and found that the number of RBCs, WBCs and lymphocytes and Hb increased; also, the average platelets decreased and effectiveness of this herb on modulation of the immune system was approved (Immunomodulatory) (16). In Shah et al. study, it was determined that toxic effect of ethanolic extract of Ruta chalepensis plant reduced the number of RBC in animals; however, this effect has not been observed in fennel plants (18).
Studies have shown that phenolic compounds isolated from aqueous extracts of fennel have the activity of absorption and removal of free radicals. The pharmacological effects of this plant can be attributed to existence of these compounds and their antioxidant effects (19). In a study to examine and verify this property of fennel, Oktay et al. showed that aqueous and ethanolic extracts of fennel seeds have the strongest antioxidant effects according to the absorption tests of anionic radicals and chelating activity of metals. Therefore, antioxidant activity of fennel plant maintains the RBC membrane against oxidizing factors and increases their life-time (20). Hanefi et al. showed that fennel seed prevents the progression of chronic liver injuries and acts as a strong hepatoprotective against hepatic fibrosis induced by carbon tetrachloride (21). On this basis, a possibility may be existed that phenolic compounds contained in this plant are a factor effective in protecting the liver with their antioxidant effect (22). Fennel herb can also increase the RBCs by affecting the liver and kidney as well as increasing the erythropoietin (15). Therefore, the present research findings in the field are consistent with the findings of previous studies and a significant increase in the average of RBC due to the fennel seed extract consumption can be justified and approved.
Other objectives of the present study were to investigate the effect of fennel on blood leukocyte count. Leukocytes constitute the most important defensive cells of blood. Leukocytes are transported by a regular circulation of blood to lymph, from the inside of the vessel to the outside and from within the tissue toward the bloods. These cells apply a full and comprehensive supervision on various parts and the proprietary tissues of the body and protect the body against pathological factors (23). In the present study, increased number of WBC was observed in the experimental group. Cherng et al. investigated the immunomodulatory effects of fennel on peripheral blood mononuclear cells and indicated that the extract of fennel has a strong stimulating effect on PBMC cells experimental models (24). Some compositions of the fennel seed extract are Trans-Anethole, Limonene, Fenchone, alpha-Phellandrene and Myrcene (25). In addition, sterols, triglycerides, flavonoids and Coumarins identified in this plant have pharmacological properties such as analgesic, anti-fever and anti-microbial effects (26). Antimicrobial properties of ethanolic extract of fennel have been proven (27-30). Anethole has antioxidant and anti-inflammatory effects and wound healing activity in experimental models (30, 31). Therefore, a significant increase in WBC by extracts of fennel seeds in this study can confirm the anti-inflammatory and anti-microbial effects of this herb.
According to the findings of the present study, fennel significantly increased coagulation time in the experimental group. Evidence indicates that compounds such as flavonoids, phenylpropanoid, phenolic and coumarin compounds have anti-thrombosis and anti-platelet effects. Based on studies, fennel by its atypical composition has anti-thrombosis effects through, vasodilatation and anti-platelet properties (32). The phenylpropanoid compounds by inhibiting arachidonic acid A and Thromboxane A and ADP demonstrated the maximum effect of anti-platelet (33). Thus, increased time of the coagulation in the present study is in agreement with previous study (31). Investigating other hematological factors did not show significant changes compared to the control group.
Probably due to liver protecting effects, vitamin C, polyphenols and antioxidant activity, fennel slows the aging process of cells and reduces the adverse effect of free radicals on the membrane of cells including red blood cells and in this way increases its count. In addition, increasing the number of white blood cells is probably due to immunomodulatory effect in the stimulation of macrophages, production of nitric oxide and ROS. On the other hand, fennel may reduce the risk of thrombosis by with regarding increasing the coagulation time and its anti-platelet effect. Other extracts of fennel can be studied using different solvents to further validate the obtained results and to investigate their hematological effects. Moreover, by preparing an appropriate formulation of effective extracts, their clinical effects can be studied in preparing proper medicinal plant for treating some blood diseases.