1. Background
Atherosclerosis is a chronic inflammatory disease that annually takes the lives of many people worldwide (1, 2). Atherosclerosis is a complex endothelial dysfunction that leads to an inflammatory response, and is induced by elevated and modified low-density lipoproteins (LDL), free radicals, oxidative stress, hypertension, or combinations of these and other factors (3, 4). A major reason for extracellular lipid accumulation in atherosclerotic plaques is binding of LDL to proteoglycan of artery wall into the subendothelial space (5, 6).
TGF-β plays a key role in the maintenance of normal blood vessel wall structure. Specific defects in the genes encoding TGF-β are linked to a range of cardiovascular syndromes including atherosclerosis (7). Studies show that TGF-β enhances proteoglycans expression in vascular smooth muscle cells and results in the formation and development of atherosclerotic plaques (6, 8).
ET-1 is a peptide consisting of 21 amino acids normally produced in physiologic conditions in small amounts by endothelial cells and is abundantly produced in pathophysiologic conditions by different cell types including endothelial cells, vascular muscle cells, and macrophages (9, 10). ET-1 is a potent vasoconstrictor and pro-inflammatory peptide that plays a role to control and maintain vascular homeostasis. Disturbance in endothelin regulation alters the normal function of vascular cells, which is correlated with the development of atherosclerotic plaques (10). ET-1 is increased in atherosclerotic plaques, as well as elevated in the serum of diabetic patients that have accelerated atherosclerosis (11). ET-1 causes an increase in LDL oxidation through stimulating reactive oxygen species (ROS) production in endothelial cells and vascular smooth muscles, on one hand, and promotes proteoglycans synthesis on the other hand, all of which ultimately lead to atherosclerotic plaques induction (5, 12).
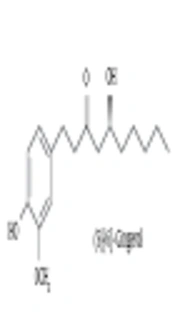
The authors’ previous study revealed that (S)-[6]-gingerol inhibits proteoglycans synthesis and has a high structural similarity with curcumin (13). In that study, the role of (S)-[6]-gingerol to inhibit TGF-β-induced biglycan synthesis. Curcumin [1,7-bis- (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the major constituent of turmeric powder extracted from the rhizomes of Curcuma longa (13, 14). Curcumin possesses anti-inflammatory, antioxidant, anti-mutagenic, and anti-carcinogenic activities (15, 16).
2. Objectives
The current study aimed to investigate the expression of ET-1 in BAECs induced by TGF-β.
The studies show that curcumin may has a potent protective effect against cardiovascular disease, specifically atherosclerosis. We show that curcumin inhibit ET-1 mRNA expression induced by TGF-β.
3. Methods
Dulbecco modified Eagle’s medium (DMEM) and penecilin/streptomycin and fetal bovine serum (FBS) were obtained from Gibco (Invitrogen, Carlsbad, CA, USA). Recombinant TGF-β was purchased from Cell Signaling (Boston, MA, USA); SB431542 and curcumin were purchase from Sigma Aldrich (MO, USA).
Bovine aortic endothelial cells (BAECs) derived from bovine aorta were kindly donated by professor Peter J Little (School of Pharmacy, The University of Queensland, Australia). BAECs were maintained in DMEM with 1 mM glucose, 10% FBS (Invitrogen, Carlsbad, CA, USA), and 1% penicillin-streptomycin solution at 37°C in 5% CO2. For the experiments, BAECs were seeded into a 70-mm petri dish and maintained until confluence. Then the cells became quiescent by serum starvation for 16 hours prior to treatment. To check the time dependency of TGF-β, BECs were treated with TGF-β (2 ng/mL and 10 ng/mL) for one, two, four, and six hours. To study the curcumin effect on the expression of ET-1 mRNA, BAECs were pretreated with SB431542 (10 µM) and curcumin (5 µM, 10 µM, and 15 µM) for 30 minutes; then TGF-β (10 ng/mL) was added and the cells were harvested after six hours.
Total cellular RNA was isolated from the cells using the RNeasy® Plus Mini Kit (Qiagene, Valencia, CA). The RNA concentration was quantified using a NanoDrop-2000 (Thermo Fischer Scientific). Also, to assess sample quality, 260/280 or 260/230 ratios were measured and considered for each sample. One microgram of total RNA was reverse transcribed with a PrimeScript™ RT Reagent Kit (TaKaRa, Dalian, China). The mRNA expression of ET-1 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; as the internal control) were quantified by quantitative real-time polymerase chain reaction (qPCR) (SYBR Green) using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems). The amplification protocol consisted of 30 seconds at 95°C followed by 40 cycles of PCR steps (five seconds denaturation at 95°C, 30 seconds annealing and extension at 57˚C). After amplification, a melting curve was acquired to confirm the absence of non-specific products and also determine Tm of each product to calculate accuracy of the study product. Real-time PCR was performed in a final volume of 20 µL of reaction mixture consisting of 10 µL SYBR Green Master Mix (Takara), 0.8 µM of each primer, and 3 µL of cDNA.
The employed primer sequences were as follows:
ET-1 forward: 5’-TCTGGACATCATCTGGGTCA-3’ and reverse 5’-CTTGGCAAAAATTCCAGCAT-3’ (17);
GAPDH forward: 5’-AGCCACATCGCTCAGACAC-3’, and reverse 5’GCCCAATACGACCAAA-TCC-3’ (18).
All the experiments were performed in triplicate. Statistical analysis was performed by one-way ANOVA with SPSS version 19 and P < 0.05 was considered as statistically significant. Normalization of data was performed to adjust a control variation between individual experiments; data were shown as mean ± standard error of the mean (SEM); curves were fitted using GraphPad Prism software.
4. Results
ET-1 is expressed in endothelial cells. To evaluated anti-atherosclerotic properties of curcumin, expression of ET-1 in cells stimulated with TGF-β was measured.
4.1. The Effect of TGF-β on the Expression of ET-1 mRNA
Quantitative real-time PCR showed that the ET-1 mRNA expression significantly increased in two and six hours following the induction by TGF-β (Figure 1A). ET-1 mRNA expression in the TGF-β-treated group was significantly higher after six hours with 10 ng/mL TGF-β compared with that of the control group (Figure 1B). ET-1 mRNA expression showed no significant changes in either groups. This experiment showed that ET-1 mRNA elevated in a dose dependent manner after stimulation with TGF-β.
TGF-β stimulated mRNA ET-1 in BAECs. A, The cells were treated with TGF-β (10 ng/mL) at course-time (1 h, 2 h, 4 h, and 6 h), in which a 6-h treatment with TGF-β significantly increased the induction of ET1 mRNA compared with the control group (**P < 0.01); B, the cells were harvested after exposure to two different concentrations of TGF-β (2 ng/mL and 10 ng/mL) for 6 h. ET-1 mRNA at 10 ng/mL of TGF-β was significantly higher than that of the control group (** P < 0.01).
4.2. The Effect of Curcumin and SB31542 on the Expression of ET-1 mRNA
The current study investigated the effect of curcumin on the expression of ET-1 mRNA. Three different concentrations of curcumin (5 µM, 10 µM, and 15 µM) were employed to identify dose dependency inhibition of curcumin on the expression of ET-1 induced by TGF-β. It was observed that TGF-β increased ET-1 expression. The impact of SB431542 (10 µM), inhibitor of TGF-β receptor, on ET-1 expression was also examined alongside of curcumin. Quantitative real-time PCR showed that the ET-1 mRNA expression significantly decreased in a dose dependent manner after exposure to curcumin (Figure 2).
Curcumin inhibited expression of mRNA endothelin-1 by TGF-β. BAECs treated with TGF-β (10 ng/mL, 6 h), curcumin (5 µM, 10 µM, 15 µM) and SB431542 (10 µM) were pretreated for 30 min before adding TGF-β. ET-1 mRNA expression significantly increased in TGF-β group than the control (##P 0.01). ET-1 mRNA expression significantly decreased in the groups exposed to curcumin and SB431542 compared to TGF-β group (*P < 0.05; **P < 0.01).
5. Discussion
The mechanism of anti-inflammatory/antioxidant activity of curcumin is not known clearly to date. Results of the current study showed that curcumin could downregulate the expression of ET-1 mRNA in BAECs when stimulated with TGF- β. TGF-β is among the factors that can induce ET-1 expression (14, 15). In agreement with this fact, the current study also demonstrated that TGF-β can significantly increase the level of ET-1 mRNA (Figure 1). ET-1 upregulation by TGF-β results in stimulate the synthesis of proteoglycans, increased free radicals and oxidative stress aggravation, the result of which is LDL oxidation, followed by oxidized low-density lipoproteins (LDLs) binding to proteoglycans, and finally resulting in atherosclerotic plaques formation (16, 19). The presence of inhibitors in each part of this pathway could be an approach to improve this disease through inhibitory impact on atherosclerotic plaques formation.
SB431542 is known as a chemical inhibitor of TGF-β receptor that significantly reduces ET-1 expression, but is not considered as a therapeutic compound, while curcumin could decrease ET-1 mRNA expression (Figure 2). Gingerol is a natural compound that inhibits proteoglycans synthesis (13). Curcumin is highly similar to gingerol regarding the chemical structure; therefore, curcumin could affect atherosclerotic plaques formation negatively both by reducing ET-1 expression through TGF-β inhibition, and proteoglycans synthesis inhibition.
Curcumin is a natural therapeutic compound mostly known for its antioxidant feature, and little research is conducted on its anti-sclerotic properties. The current study results indicated that this compound could prevent increased ET-1 stimulated by TGF-β. So, curcumin through inhibition of ET-1 expression may lead to decreased formation and development of atherosclerotic plaque.
Nicholson et al. showed that dietary polyphenols (resveratrol and quercetin) decreased the expression of Et-1 in HUVEC (human umbilical vein endothelial cells) (20). In another study, Virdis et al. found that ET-1 expression was higher in small artery of the subjects with obesity than those of the control; they also indicated that tumor necrosis factor-alpha (TNF-α) associated with imbalance of ET-1/nitric oxide in small arteries of the subjects with obesity (21).
In contrast with the current study results, Farhangkhoee et al. showed that curcumin upregulated ET-1 in diabetic rats (22). Jane Chiu et al. investigated the role of curcumin to prevent abnormality due to diabetes; they showed TGF-β and ET-1 induced in diabetic rats and then curcumin significantly inhibited the expression of TGF-β and ET-1 (23).
How curcumin inhibits TGF-β or how it prevents proteoglycans synthesis are among the questions, which require more studies to respond, and the current study was an introduction to more studies in order to determine the antiatherosclerotic mechanisms of curcumin.
In spite of the current study limitation to use bovine cells that differ from those of human, other studies and also the experiments in authors’ lab showed that these cells were closely correlated with human cells.
5.1. Conclusion
Curcumin is known for its antioxidant properties. In previous studies the anti-inflammatory and anti-atherosclerotic mechanisms of curcumin were not clearly described. The current study revealed that curcumin could prevent enhancements of ET-1 expression stimulated by TGF-β and showed anti-atherosclerotic properties of curcumin.