1. Background
Ficus religiosa L. is known as an important biofuel wood, and medicinal tree that belongs to the family of Moraceae and it distributes in the wild or cultivated types throughout India, Pakistan, Bangladesh, Nepal, and Southern Iran. Ficus religiosa has intense religious holdings among Buddhists and Hindus (1, 2). It has a tipped, leathery, long petiolate, and heart-shaped leaves. It is also known as a popular tropical shade tree due to its easy adaptability to various soil conditions and well-formed shape. Ficus religiosa L. also has an ornamental value and is commonly employed as a roadside tree. Its tender leaf buds and fruits are sometimes consumed at the times of famine. Birds also eagerly eat the fruits (3). Furthermore, F. religiosa L. has a significant medicinal value (4). For instance, the bark extract is employed for treating various diseases, such as inflammations, glandular swelling, diabetes, stomatitis, asthma, wound healing, and skin diseases. Leaf extracts are applied for treating constipation, and ear problems (5), and root barks have a positive effect in cleaning ulcers and leucorrhoea (6). Moreover, stem barks have been recently reported to have a remarkable acetylcholinesterase inhibitory activity that is useful for treating Alzheimer’s disease (7). There are some identified secondary metabolites, including methyl oleanolate, caffeic acid, stigmasterol, bergenin, lupin 3-one, lanosterol, noctacosanol, flavonoids, amides, and β-sitosterol, which are responsible for different biological activities (8). However, some of the secondary metabolites from F. religiosa have remained unexplored (4). The isolation and characterization of such metabolites are often conducted through naturally occurring sources. Such processes are time and labor consuming and affected by different environmental conditions. Hence, there is a dire need for providing an alternative method to isolate secondary metabolites of this valuable medicinal germplasm (9). In the mid 1960s, the cell culture of the plant was introduced as a useful tool for studying and producing secondary metabolites of the plant. Some of the important secondary metabolites, employed in pharmaceuticals, cosmetics, and food industry, have been produced through cell, callus, root, and shoot cultures of the plant (4, 10, 11). The advantages of direct manipulation of the cell, tissue, and organ cultures are more than the traditional isolation of secondary metabolites. It has been reported that such manipulations increase the production of secondary metabolites in many species (12). The rate success amongst in vitro culture of woody plants, especially old age plants, has remained a challenging task, despite great development in plant tissue culture techniques (9, 12). The age of the explant could play a significant role in plant tissue culture, and physiologically younger segments had a better performance than the older one (13). One way to eliminate the effect of plant’s age is to use different parts of in vitro-grown seedlings (14). Moreover, the response of plant growth regulators that change during plant tissue culture can be varied from species to species and also this response depends on the ability of tissues to respond and the types of plant growth regulators (15). For instance, auxins and cytokinins play an essential role in plant tissue culture in the procedures for plant propagation, such as shoots development and stimulation, and callus formation and induction (16).
2. Objectives
The present study investigated the optimized conditions for in vitro seed germination and callus formation via different parts of in vitro-grown seedlings. The callus culture generated in this study could be easily utilized for better screening, isolation, and characterization of different secondary metabolites of F. religiosa for future applications in pharmaceuticals.
3. Methods
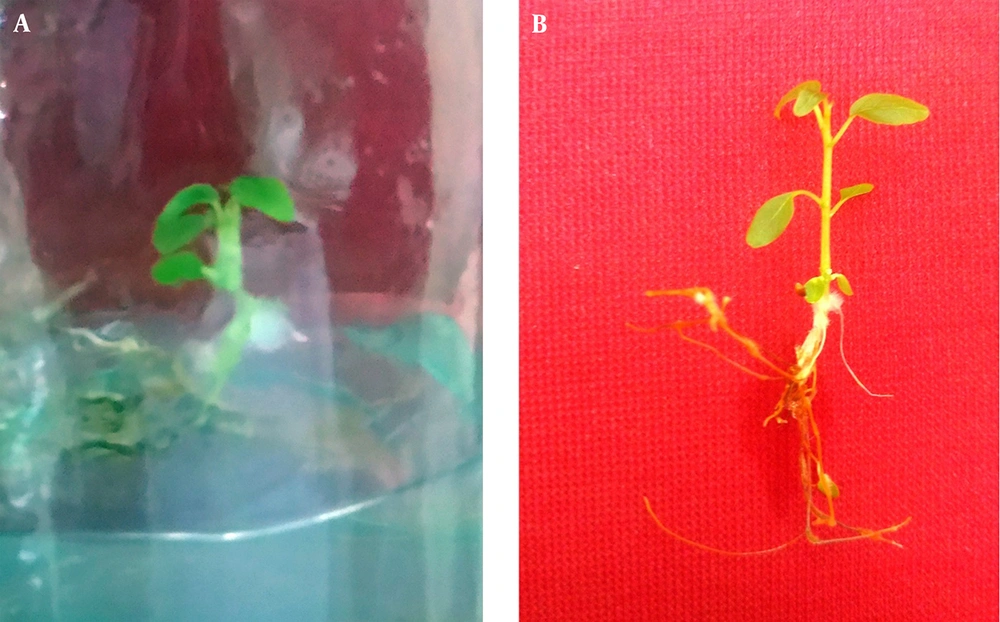
The fruits of F. religiosa were collected from a 45- to 50-year-old F. religiosa mother plants that were grown on the campus of Ramin Agriculture and Natural Resources University, Khuzestan, Iran. The fruits were washed for 30 minutes under tap water and rinsed 5 to 6 times. Then another washing process was applied using a liquid soap solution followed by rinsing with tap water again. Further surface sterilization treatment was carried out in a laminar air flow chamber. The surface of the seeds was sterilized for 10 seconds with 70% aqueous ethanol, and dipped in 10% bleach solution (with 5% active NaOCl) for 5 minutes and finally washed 3 times with sterilized distilled water. A factorial experiment was set to estimate the effect of different strengths of MS medium (one-tenth strength of MS, half strength of MS, and full strength of MS) and light condition (24-hour light and dark) on in vitro seed germination of F. religiosa. Media were supplemented with 3% w/v sucrose, pH was adjusted to 5.8 with 1 N HCl or KOH, solidified with 0.6% agar and autoclaved at 121°C for 15 to 20 minutes at 15 psi or one bar pressure, and poured into the culture bottles (2 cm in diameter and 15 cm in height). All cultures were kept in a culture room at 26 ± 2°C under light (63 μmol m-2s-1) or dark conditions. The seeds were germinated after 14 days (Figure 1A), and seedlings from in vitro germinated seeds were utilized as the source of explants for the next experiment. The cultures were observed periodically, and germinated seeds were counted as well. The seeds representing radicle protrusion were assumed germinated. At the end of this experiment after 14 days, percentage of seed germination was calculated. Explants with 0.5 to 1.0 cm of length, from the leaf, petiole, internode, and root of 1-month-old seedlings (Figure 1B) were cultured in MS medium consisting of various concentrations of plant growth regulators for callus induction (Table 1). The cultures were inoculated at 26±2°C in darkness. The frequency (%) of callus formation and its fresh weight were recorded after 4 weeks of culture. The experiments were conducted with a Completely Randomized Design (CRD). There were 10 replicates per treatment, and each treatment was repeated in 3 sets. Data analysis was set up through Analysis of Variance (ANOVA) followed by Duncan’s multiple range test. Data were analyzed by SAS version 9.3.
| Treatment | Seed Germination, % |
|---|---|
| Light conditions | |
| Light | 67.778 a |
| Darkness | 55.556 b |
| Different strength MS medium | |
| 0.1 strength MS | 78.333 a |
| 0.5 strength MS | 68.333 b |
| Full strength MS | 38.333 c |
| Different strength MS × Light conditions | |
| 0.1 strength MS × Light | 83.333 a |
| 0.1 strength MS × Darkness | 73.333 b |
| 0.5 strength MS × Light | 76.667 ab |
| 0.5 strength MS × Darkness | 60.000 c |
| Full strength MS × Light | 43.333 d |
| Full strength MS × Darkness | 33.333 e |
aMeans in each column followed by same letters at superscript are not significantly different according to DMRT at P < 0.05.
4. Results
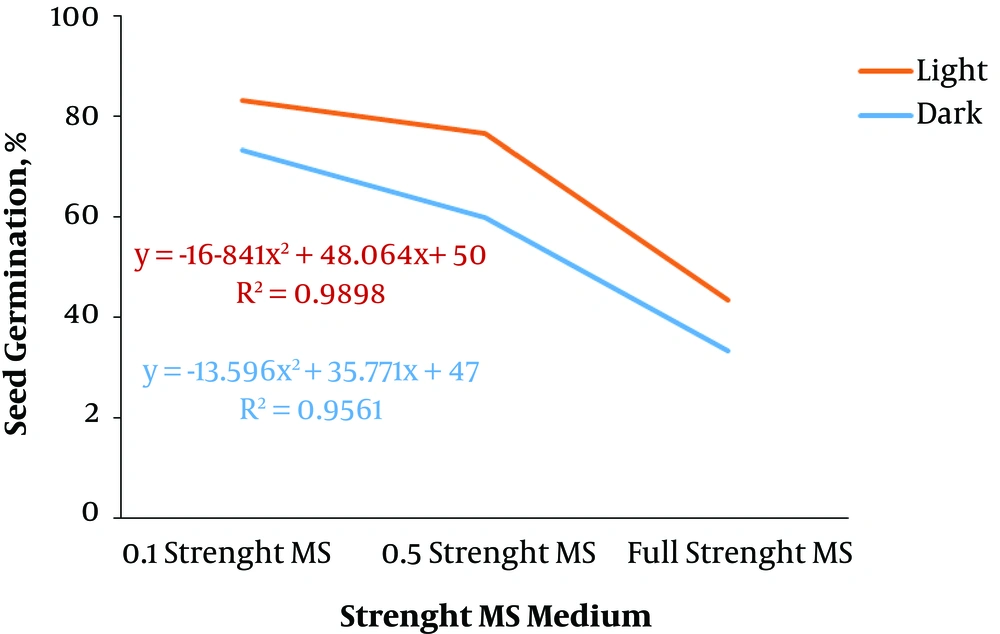
According to the current results, one-tenth strength MS medium and light (83.33%) had the highest seed germination percentage among other treatments while it did not have a significant difference in the half-strength MS medium and light condition (Table 1).
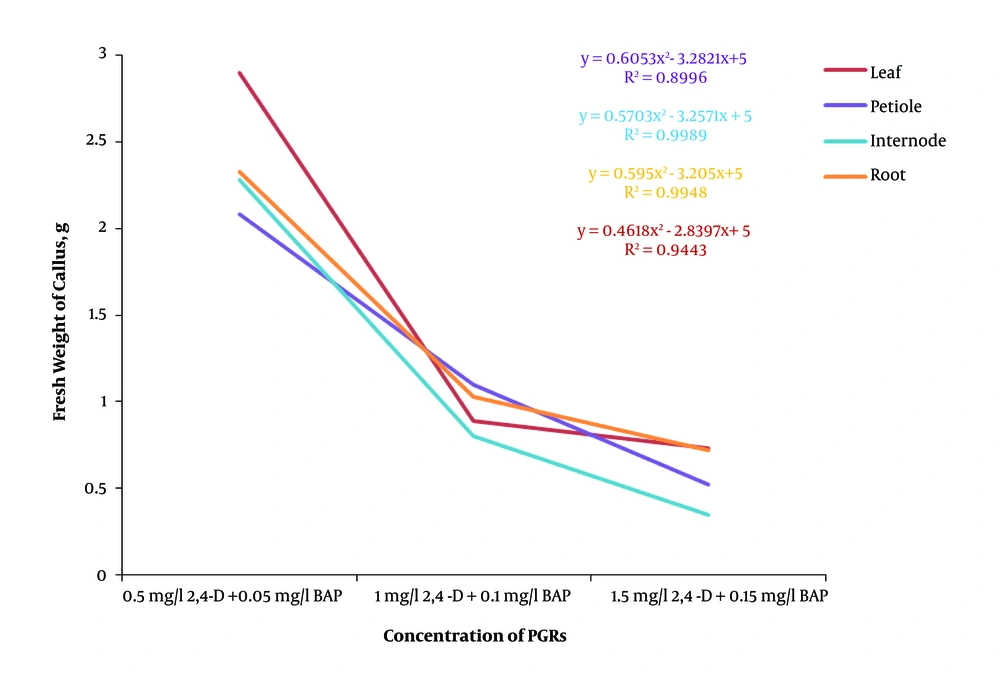
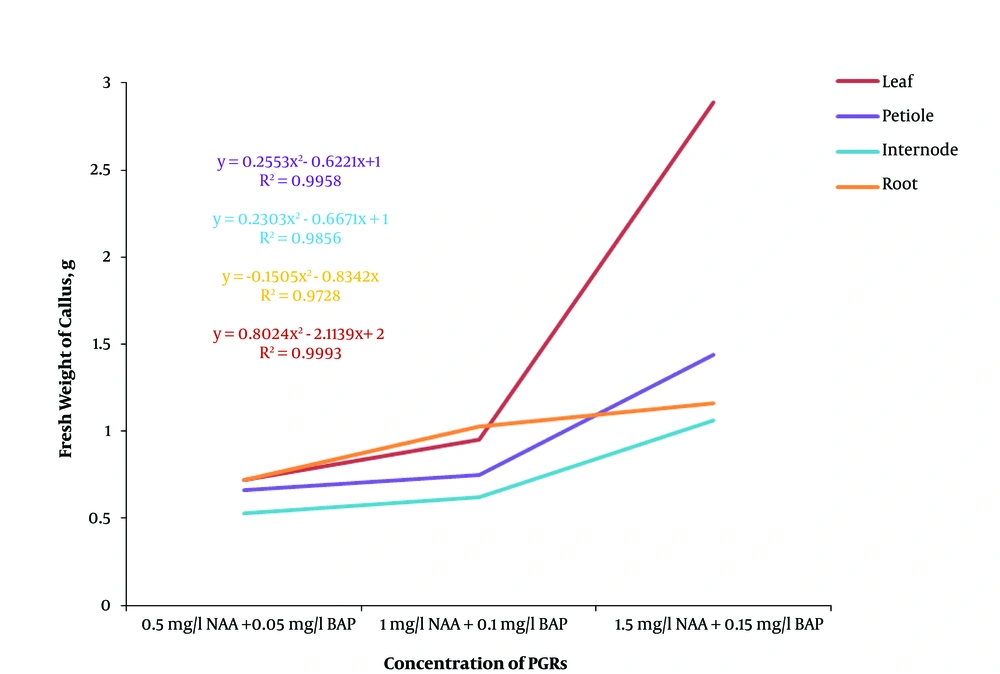
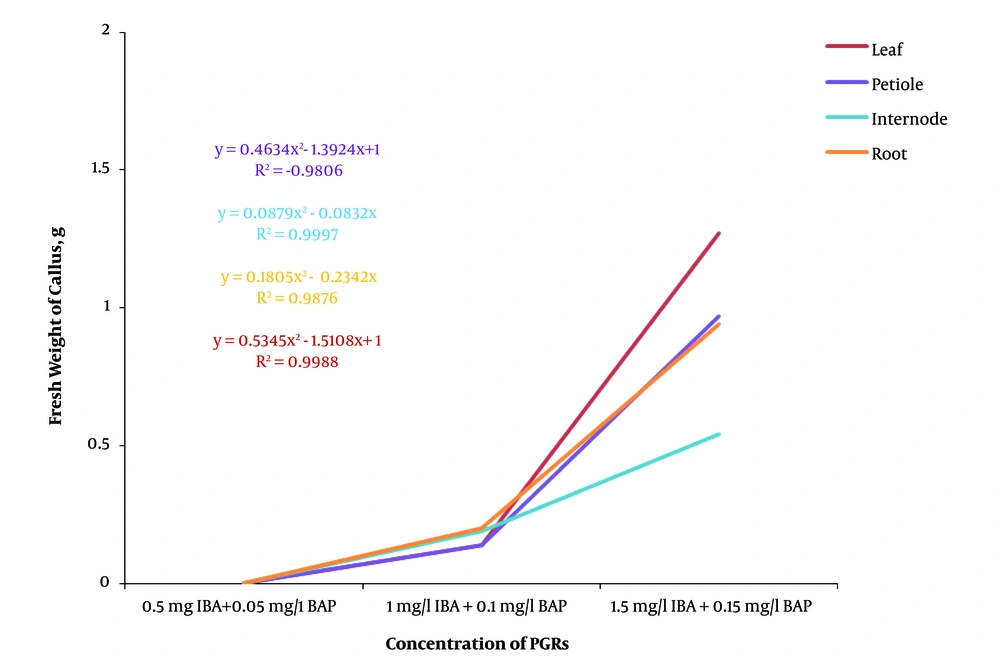
Generally, the regression analysis indicated that there was a decreasing polynomial trend from 0.1 strength MS to full strength MS in both dark and light conditions and the light condition had a higher R2 than the dark condition (Figure 2). The effect of different concentrations of plant growth regulators on callus induction from F. religiosa was studied in leaf, petiole, internode, and root explants that were cultured in MS medium (Table 2). The explants did not show any response to control treatment (MS medium without any PGRs) and initially the cut ends, and later, the whole explant blackened because of phenolic exudation, and finally the explants lead to death within 10 days of culture. After 7 days of culture, an initial response, such as curl and swelling, of explants was observed. After 2 weeks of culture, the callus induction was begun from the cut margins of explants that were cultured in MS medium containing with 2, 4-D, IBA and NAA along with BAP, in the absence of light (Table 2). A wide range of callus induction frequencies were observed at various concentrations of PGRs, and also a high callus induction was obtained in 2,4-D, NAA, and IBA along with BAP, respectively. However, there was no callus formation observed at low concentrations of IBA (0.5 mg/L) along with BAP (0.05 mg/L). According to the regression analyses in Figure 3, the highest fresh weight was obtained from 0.5 mg/L 2,4-D and 0.05 mg/L BAP in explants, and also 2, 4-D produced yellow-brownish friable callus from all explants (Figure 4). Based on Figure 3, by increase in the concentration of 2,4-D, the callus induction decreased significantly. However, Figure 5 indicated that increasing in NAA concentration induced more callus formation and the highest callus formation was achived in explants derived from leafs at high concentrations of NAA. Same as NAA, callus formation had an incresing polynomial trend with incresing concentration of IBA (Figure 6).
| Plant Growth Regulator, mg/L | Leaf | Petiole | Internode | Root | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,4-D | NAA | IBA | BAP | Callus Formation Frequency (%) | Fresh Weight of Callus, g | Callus Formation Frequency (%) | Fresh Weight of Callus, g | Callus Formation Frequency (%) | Fresh Weight of Callus, g | Callus Formation Frequency (%) | Fresh Weight of Callus, g |
| - | - | - | - | 0.00 f | 0.00 f | 0.00 e | 0.00 h | 0.00 d | 0.00 h | 0.00 d | 0.00 f |
| 0.5 | - | - | 0.05 | 100.00 a | 2.90 a | 100.00 a | 2.08 a | 100.00 a | 2.28a | 100.00 a | 2.33 a |
| 1.0 | - | - | 0.1 | 93.33 ab | 0.89 c | 90.00 b | 1.10 c | 96.67 a | 0.80 c | 100.00 a | 1.03 c |
| 1.5 | - | - | 0.15 | 83.33 c | 0.73 d | 53.33 c | 0.52 f | 43.33 c | 0.35 f | 53.33 c | 0.72 d |
| - | 0.5 | - | 0.05 | 33.33 e | 0.72 d | 83.33 b | 0.66 e | 83.33 b | 0.53 e | 96.67 a | 0.72 d |
| - | 1.0 | - | 0.1 | 86.67 bc | 0.95 c | 90.00 b | 0.75 e | 93.33 a | 0.62 d | 100.00 a | 1.03 c |
| - | 1.5 | - | 0.15 | 100.00 a | 2.89 a | 100.00 a | 1.44 b | 100.00 a | 1.06 b | 100.00 a | 1.16 b |
| - | - | 0.5 | 0.05 | 0.00 f | 0.00 f | 0.00 e | 0.00 h | 0.00 d | 0.00 h | 0.00 d | 0.00 f |
| - | - | 1.0 | 0.1 | 56.67 d | 0.14 e | 26.67 d | 0.14 g | 36.67 c | 0.19 g | 50.00 c | 0.20 e |
| - | - | 1.5 | 0.15 | 93.33 ab | 1.27 b | 90.00 b | 0.97 d | 83.33 b | 0.54 e | 83.33 b | 0.94 c |
aMeans in each column followed by same letters at superscript are not significantly different according to DMRT at P < 0.05.
A, Yellowish and compact callus induction on MS + 1.5 mg/L NAA + 0.15 mg/L BAP derived from root segments; B, Greenish and compact callus induction on MS + 1.5 mg/L IBA + 0.15 mg/l BAP derived from leaf segments; C, Yellow-brownish and friable callus induction on MS + 0.5 mg/L 2,4-D + 0.05 mg/L BAP derived from internode segments; D, Yellowish and compact callus induction on MS + 1.5 mg/L NAA + 0.15 mg/L BAP derived from leaf segments, bar = 0.5 cm.
Furthermore, MS medium consisting of IBA produced greenish compact calli from explants while NAA produced yellowish compact calli (Figure 4).
5. Discussion
The overall objective of the first experiment was to study the effect of light and dark condition with various strengths of MS medium in in vitro seed germination and to investigate callus formation from the leaf, petiole, internode, and root segments of F. religiosa. The rate of F. religiosa seed germination increased exponentially under the light condition. Despite that there are no reports on in vitro seed germination of F. religiosa, the effect of light on increasing seed germination of other plants has been investigated in many studies (17, 18). There are also many studies that have proved the effect of higher light intensity in promoting seed germination (19). Similar to the current results, Wall et al. (20) indicated that seed germination was higher under the light condition when compared with the dark condition in Pyxidanthera brevifolia. Furthermore, Oh et al. (21) elucidated that light could activate the degradation of PIL5 protein to induce seed germination via gibberellic acid in Arabidopsis. Similar effects of various strengths of MS medium on seed germination have been described for various species (22, 23). Martendal et al. (24) applied water-agar medium in order to obtain the highest germination frequency for Byrsonima cydoniifolia. Since the previous reports proved the inhibitory nature of MS medium on seed germination of Aconitum heterophyllum, seed germination occurred on water-agar medium (25). Furthermore, the 0 and ½ strength of MS media in comparison with full strength MS medium, produced a higher seed germination rate in Rauvolfia serpentina (26). In another report, Samuel et al. (27) indicated that the reduction of full Strength MS medium to half strength MS medium was adequate for enhancing the seed germination frequency of Givotia rottleriformis. When embryos are autotrophic, seed germination might occur in media without any nutritional elements and sucrose, by eliminating its supplemental energy source (24). On the other hand, full MS medium caused delay in seed germination due to high nutrition concentrations that were above the threshold level for seed germination (27). However, in other studies conducted by Solanki and Siwach (28) and Hesami and Daneshvar (11), it became clear that the response of seeds to various salt concentrations were different from one species to another species. Overall, seed germination rate was a paramount element in developing in vitro tissue culture, especially in producing a uniform set of seedlings.
In the second experiment, the effect of various concentrations of PGRs on callus formation from 4 different explants of F. religiosa was investigated. The plant growth regulators played an important role in callogenesis response under in vitro conditions. Many studies proved the positive effect of 2,4-D and NAA in callus induction (14, 29). According to the current results, 2,4-D and NAA along with BA had the highest callus formation frequency among other Auxins, which is in line with the study of Siwach et al. (9), which indicated that MS medium containing 0.5 and 1.0 mg/L 2,4-D produced acceptable callus formation from nodal, internodal, and shoot apices explants of F. religiosa. Also, Narayan and Jaiswal (30) reported that the highest callus formation from leaf explant of F. religiosa was achieved on MS medium supplemented with 0.5 mg/L NAA plus 1.0 mg/L BAP. Moreover, Su et al. (31) indicated that a high ratio of auxin/cytokinin is critical for callus formation. 2,4-D acts as herbicide, and callus formation could be limited to a high concentration of this auxin (14). However, Parasharami et al. (32) reported that MS medium consisting of 2.4 mg/L 2,4-D plus 1.0 mg/L BA was the best treatment for callus induction from fruit segments of F. religiosa. In the current study, various types of callus were obtained from various concentrations of PGRs. Also, 2, 4-D treatment produced a friable callus, and according to previous results, the best type of callus for suspension culture and cell culture is the friable one (33). Calli usually have 2 types, including compact and friable. These types may be modified by genetic, epigenetic, and culture changes. It is also feasible to obtain various calli types from the same explants (14). Likewise, Garcia et al. (34) indicated that MS medium containing NAA, 2,4-D, and Picloram (PIC) produced friable and compact calli from the leaf, nodal, and internodal segments of Passiflora suberosa. Karami et al. (35) concluded that various calli types from Elaeagnus angustifolia obtained from different concentrations of PGRs. In contrast, callus morphology in Hildegardia populifolia was similar under different growth regulators (36).
5.1. Conclusions
This is the first protocol for in vitro seed germination and callus induction from immature explants of F. religiosa. The present study provided a reliable method for forming callus via leaf, petiole, internode, and root explants. A suitable calli response was obtained in the MS medium supplemented with 2,4 -D along with BA in all explants. Since friable callus has a significant impact on suspension and cell cultures, this treatment produced the highest friable callus among other treatments. There are limited reports on the secondary metabolites of F. religiosa, thus this protocol can be employ as an alternative source of isolation, identification, and characterization of secondary metabolites at the commercial level. Further studies could be conducted for increasing secondary metabolites and identifying novel approaches in order to utilize this plant for medicinal aims.