1. Background
Withania coagulans, also known as paneerbad in Iran, is a traditional plant belonging to the Solanaceae family that possesses diverse pharmacological characteristics (1). Two active steroidal lactones in Withania root extract are withaferin and withanolides (2). Some of therapeutics effects of Withania coagulans extract (WCE) have been reported by researchers (3-5). Upadhyay and et al. reported anti-hyperglycemic effect of WCE in diabetic patients (1). Another study revealed anti-hyperlipidemia effects of WCE in rats (6). It has been reported that Withaferin A has protective effects against Alzheimer’s disease (7). Another study has shown Withania extract protects hippocampus neurons against stress (8). Shukla et al. proposed that WCE improves the liver antioxidant activity in hyperlipidemic rabbit model (9). In addition, we have shown that the administration of WCE with a high antioxidant effect protects pyramidal cells against ischemia reperfusion (10). Several studies have confirmed that herbal medicines by their antioxidant and anti-inflammatory effects attenuate the brain stroke insults (11, 12).
Brain stroke is a condition resulted from immediate depletion or interruption of blood flow in part or whole of brain that can cause deprivation of brain tissue from oxygen and glucose (13). Reperfusion to the previous ischemic brain tissue causes overproduction of reactive oxygen species (ROS) and subsequently, neuronal degeneration occurs (14). High level of oxidative activity and low concentration of antioxidant enzymes predispose the brain to oxidative stress (15). Oxidative stress can lead to the production of ROS and mitochondrial dysfunction that is an important event in cell death subsequent to brain ischemia (16). It has been widely recognized that hippocampus and striatum are more susceptible to oxidative stress (17). Thus, it seems that increasing the antioxidant defense of tissues could prevent oxidative damage following reperfusion injury.
2. Objectives
Therefore, this research was designed to evaluate the pretreatment effects of WCE on oxidative status and histopathological alteration induced by I/R in the striatum area in a rat model.
3. Methods
3.1. Plant Extract Preparation
Collection and preparation of plant root samples were done from the medicinal plant garden of the agricultural research of Sistan and Baluchestan University (Zahedan, Iran). The Withania coagulans roots were washed, shade-dried, and then mechanically crushed. To prepare the aqueous extract, 1 g of powdered samples was soaked in 20 mL methanol and water (3:1) at room temperature for 24 hours The solution was filtered by Whatman No.1 paper and evaporated in oven at 37°C to dryness. The extract was stored under refrigeration (10).
3.2. Animals
This study was carried out on 48 adult male Wistar rats (weighing 220 - 250 g). The animals were housed under a controlled environment with free access to food and tap water.
The animals were sorted randomly into four groups (n = 12):
1- Control (not receiving any medication or procedure)
2- Sham (receiving distilled water + surgical procedures without brain ischemia)
3- I/R (receiving distilled water + brain ischemia reperfusion)
4- WCE + I/R (receiving Withania coagulans extract 1000 mg/kg + brain ischemia reperfusion)
The effective dose (1000 mg/kg) of plant extract was selected based on previous studies (18, 19).
Rats received WCE or distilled water (vehicle) by feeding cannula for 30 days before ischemia.
Global brain ischemia was performed by occlusion of both common carotid arteries for 30 minutes and reperfusion was done for 72 hours (20).
Three days after ischemia, half of the animals in each group were sacrificed by overdose of chloroform anesthesia. Then, the brain was removed and rinsed with cold PBS. Next, striatum was dissected out and frozen (at -80°C) until biochemical analysis.
The brain of other subset animals was fixed in 4% paraformaldehyde solution under cardiac reperfusion after deep anesthesia by ketamine and xylazine (10:1). The brains were dissected out, postfixed in the same fixative at room temperature for 24 hours and paraffin embedded. 5 µm sections were obtained from striatum and stained with cresyl violet (Nissl) for histopathological examination, and TUNEL analysis was performed for detecting the apoptotic cells.
The present research was reviewed and approved by the animal care ethics committee of Zahedan University of Medical Sciences (EC/93/ 6883).
3.3. Biochemical Analysis
For biochemical evaluation, striatum was homogenized (1/10, w/v) in cold phosphate buffer (pH 7.4) and centrifuged at 12000 g at 4°C for 20 minutes. MDA level, GPx, SOD, and CAT activity were measured in supernatants. The protein concentration in the striatum supernatant was determined according to the method described by Bradford (21).
3.3.1. MDA Activity Assay
The amount of lipid peroxidation was indicated by measurement of Malondialdehyde in brain homogenates as described by Ohkawa et al. Briefly, 0.2 mL of striatum homogenate was added to 1.5 mL of 20% acetic acid, 0.2 mL of 8.1% sodium dodecyl sulphate, and 1.5 mL of 0.8% thiobarbituric acid. The mixture was boiled at 95°C for 60 minutes and 5 mL of n-butanol was added after cooling. The mixture was centrifuged and the absorption of the supernatant was read at 532 nm. The result obtained was expressed as nanomole/mg of protein (22).
3.3.2. SOD Activity Assay
The striatum superoxide dismutase (SOD) activity was determined according to the method described by Kakkar et al. In short, the reaction mixture was composed of striatum supernatant (0.1 mL), sodium pyrophosphate buffer (52 mM, pH: 8.3), nitroblue tetrazolium (0.3 mL, 300 µM), and phenazine methosulphate (0.1 mL, 186 µM). The reaction was initiated by addition of NADH solution (0.2 mL, 750 µM). Incubation was done for 90 seconds, and glacial acetic acid (0.1 mL) was added to stop the reaction. After adding n-butanol (2 mL), the mixture was centrifuged at 4000 g for 10 minutes. The absorbance changes at 560 nm were measured spectrophotometrically. The result of SOD activity was expressed as units/mg protein (23).
3.3.3. CAT Activity Assay
Catalase activity was measured according to a method described by Goth et al. Briefly, striatum supernatant (0.2 mL) was mixed with H2O2 (1 mL, 65 µM) for 1 minutes. Then, ammonium molybdate (1 mL of 32.4 mM) was added and the absorbance of mixture spectrophotometrically was read at 450 nm. The activity was expressed in units/mg protein (24).
3.3.4. GPx Activity Assay
Glutathione peroxidase activity was determined as previously described (25). Briefly, the mixture was consisted of striatum supernatant (10 µL), potassium phosphate buffer (0.89 mL, 100 mM, pH 7.0), EDTA (1 mM), NaN3 (1 mM), NADPH (0.2 mM), GSH reductase (1 U/mL), and GSH (1 mM). H2O2 (100 µL, 2.5 mM) was added to start the reaction and the oxidation of NADPH to NADP was determined at 340 nm. GPx activity was recorded as units/mg protein.
3.4. Histopathological Study
The sections were dewaxed by xylene, dehydrated in descending alcohol series, and then stained by 0.1% Cresyl violet acetate. The mean of the total number of intact neurons was counted in the different groups at 400 × magnification. The apoptotic striatal cells were detected by using terminal deoxy nucleotidyl transferase mediated UTP end labeling (TUNEL) staining. For this purpose, the sections were treated by xylene and graded ethanol solutions to water for paraffin removal and dehydration. The striatum sections were incubated in proteinase K (10 - 20 µg/mL) for 30 minutes, followed by 10 minutes incubation in 3% of H2O2/methanol. After that, the tissue sections were incubated by using an in situ death cell detection kit, POD (Roche, Germany) for 1 hours at 37°C and then incubated with POD for 15 minutes at 37°C. Color development was performed in the dark with DAB for 15 minutes and counter stain was done by hematoxylin solution. The number of TUNEL positive neurons was counted carefully in five sections per animal under light microscope with 400 × magnification (Olympus, Hamburg, Germany). Cell counts from the striatal on each of the five sections were averaged to provide the mean value (10).
3.5. Statistical Analysis
The data are shown as mean± SEM. One-way analysis of variance (ANOVA) was used followed by post-hoc Tukey’s test using SPSS version 16.0. P < 0.05 was considered to indicate statistical significance.
4. Results
4.1. Antioxidant Activities Estimation
4.1.1. The Effect WCE on MDA Concentration
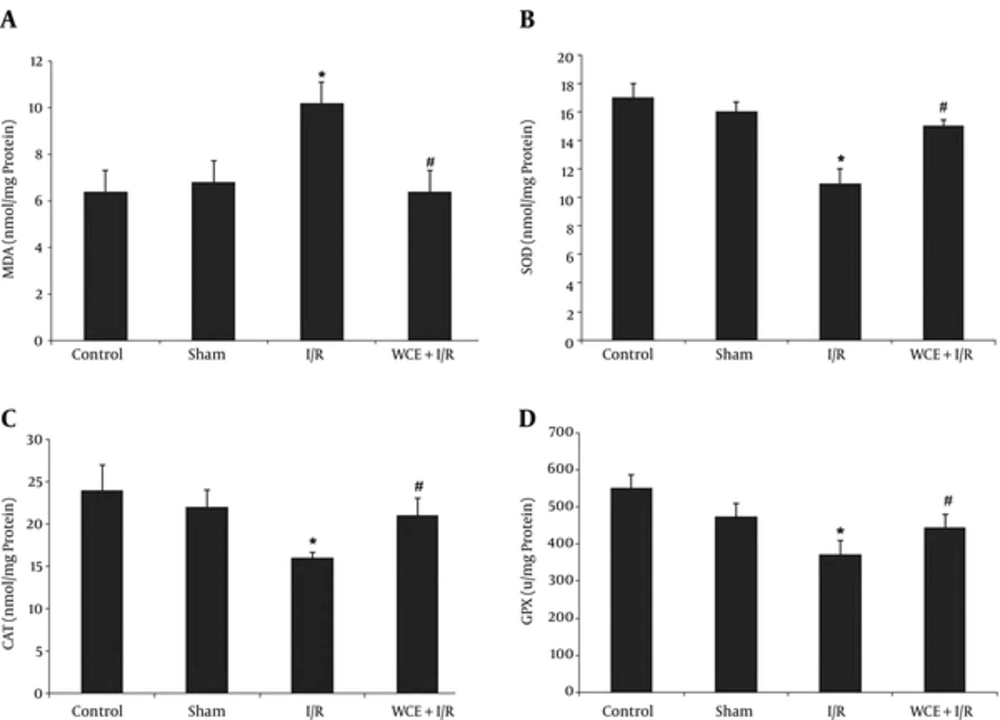
According to Figure 1A, there was an increase in MDA concentration of the striatum in I/R group (P < 0.001) in comparison with the control group. Pre ischemic administration of WCE for 30 days caused a significant reduction in MDA concentration as compared to the I/R group (P < 0.001).
4.1.2. The Effect WCE on SOD Activity
As shows in Figure 1B, the SOD activity significantly decreased (P < 0.001) in the striatum in I/R group as compared to the control group. However, the decrease in SOD activity was significantly restored by WCE pretreatment (P < 0.001).
4.1.3. The Effect WCE on CAT Activity
The results presented in Figure 1C show CAT activity significantly decreased (P < 0.001) in the striatum in I/R group as compared to the control group. Administration of WCE 1000 mg/kg for 30 days before I/R induced a significant increase in catalase activity in comparison with the I/R group (P < 0.001).
4.1.4. The Effect WCE on GPx Activity
According to Figure 1D, GPx activity significantly decreased (P < 0.001) in the striatum in I/R group as compared to the control group. However, the decrease in GPx activity was significantly restored by WCE pretreatment (P < 0.001).
4.2. Histological Assessment
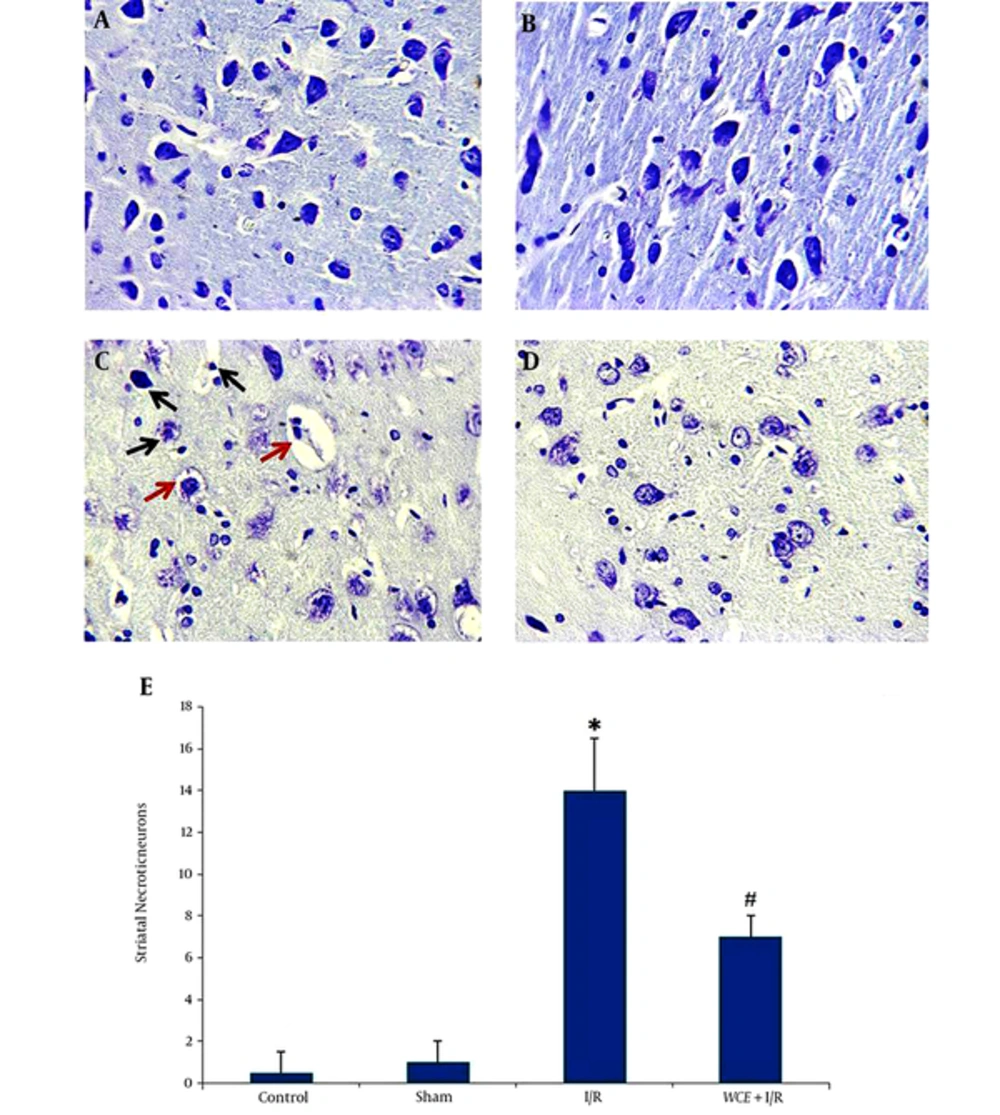
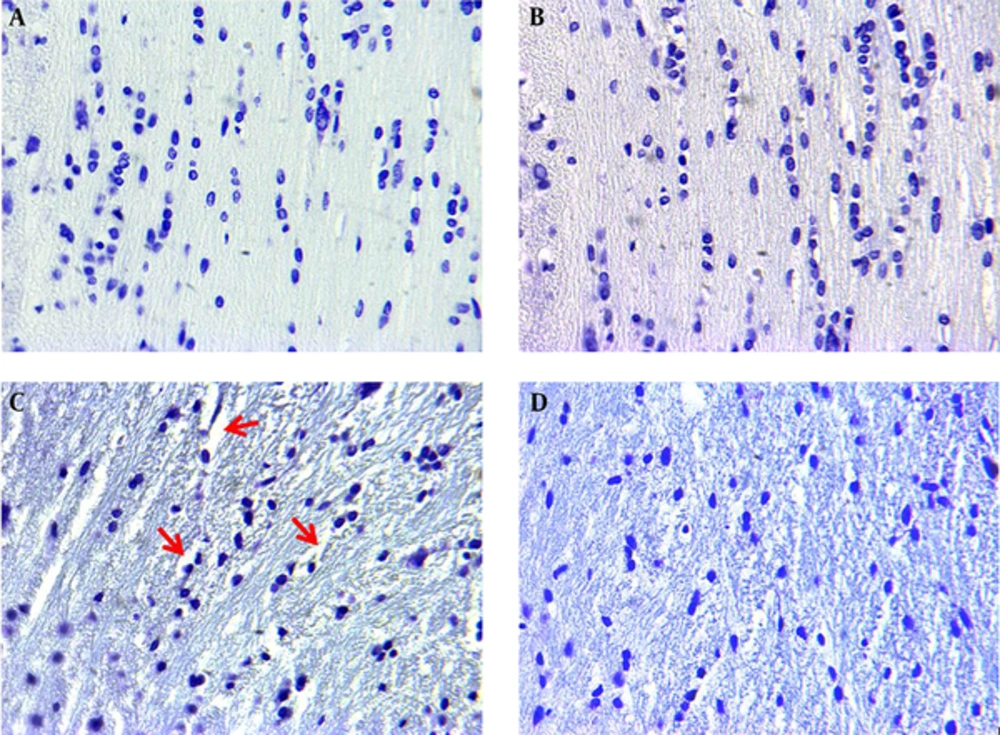
Histological study on control and sham group did not show any degenerated cells in the corpus striatum (Figure 2A and B). As shown in Figure 2C, there was a highly significant increase in the mean number of degenerated (Necrotic) cells in the corpus striatum and demyelination and axonal fiber injury in I/R group compared to the control group (Figure 2C and Figure 3C). Preischemic administration of WCE markedly decreased the number of degenerated cells in striatum (Figure 2D and E) (P < 0.001) and reduced demyelination of white matter (Figure 3D).
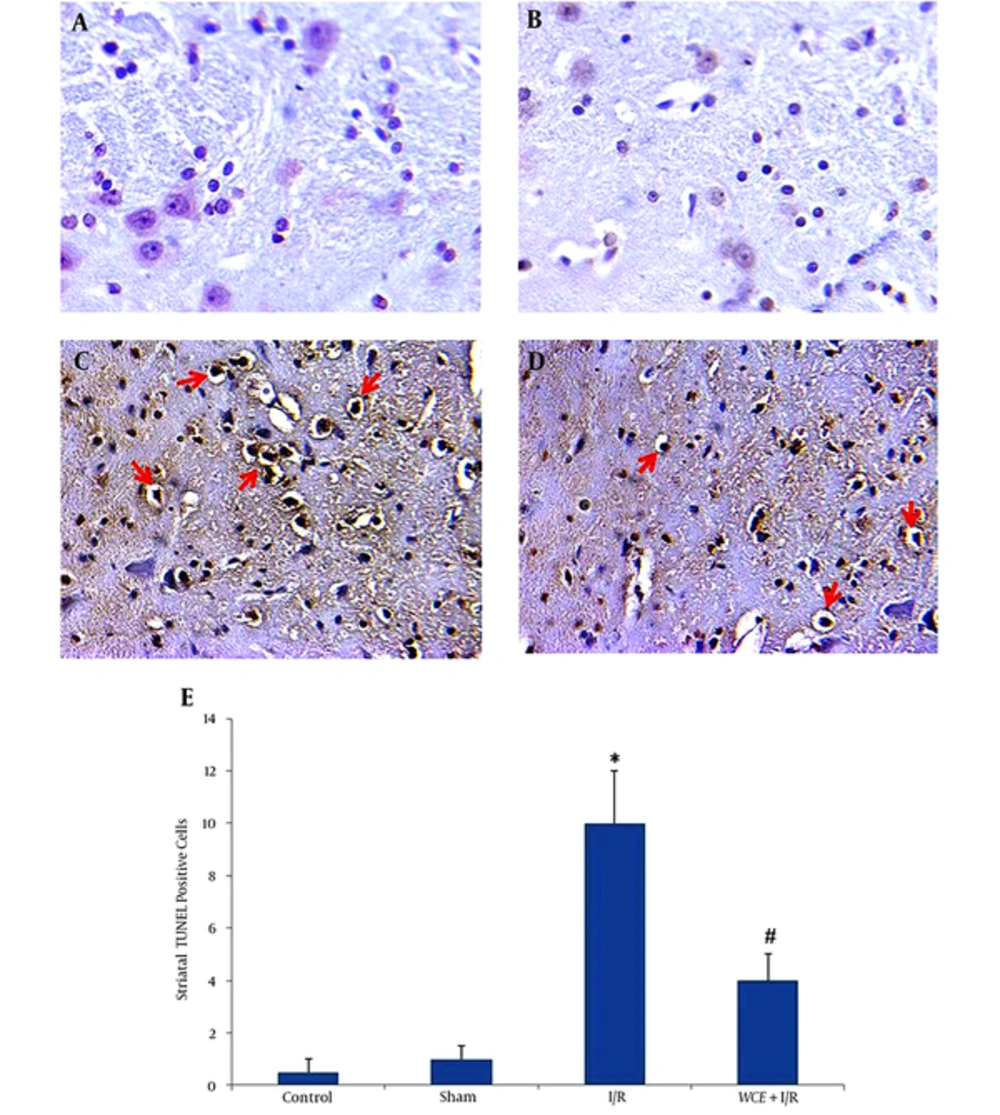
As seen in Figure 4A and B, the number of apoptotic cells in control and sham groups was negligible. There were abundant TUNEL positive (apoptotic) cells in I/R group (Figure 4C and E). Pretreatment with WCE significantly decreased TUNEL positive cells in WCE+I/R group compared to control and sham groups (Figure 4D and E) (P < 0.001).
5. Discussion
In the current study, we demonstrated that Withania coagulans root extract has neuroprotective effects against oxidative damages following global brain ischemia in rats. Our results showed that WCE improved antioxidant enzymes activity and decreased neuronal death in the rat’s striatum in global model of ischemia inducing the oxidative stress and neuronal damage.
After short time of global brain ischemia, neuronal degeneration occurs in susceptible brain regions such as hippocampus and striatum, resulting in metabolic and enzymatic changes in brain tissue (26). These changes occur due to ROS generation and thereby, yielding neuronal death (27). The brain is composed of many polyunsaturated fatty acids (PUFAs) and it is sensitive to oxidative damage, ROS attack to biological molecules like DNA, proteins, and lipids in neuronal membrane and induced neurodegeneration (28). Therefore, improvement of antioxidant enzyme activities may help prevent neuronal damage. It has been accepted that Withania coagulans has strong antioxidant effects at the effective dose of 1000 mg/kg (18, 19) for investigation of its effects on antioxidant status and striatal neurons after brain ischemia.
Malondialdehyde (MDA) is one of the lipid peroxidation products, which increases in ischemia-reperfusion. In our study, I/R induced enhancements in MDA level of striatum as shown also by others authors (29, 30) while pretreatment with WCE markedly decreased MDA level in the striatum region that is in line with Chaudhary study who showed that pretreatment with Withania somnifera (another herb from this family) inhibited lipid peroxidation (LPO) in I/R in a rat model (18). In addition, Shukla et al. showed fruit extract of Withania coagulans decreased LPO in liver of hypercholesterolemic rabbit model, which points to antioxidant properties of Withania plant (9). It has been found that 1000 mg/kg of Withania extract reduces LPO level in arthritis induced in rat (31).
Striatum is highly susceptible to oxidative damage and the current study revealed that the striatum SOD and CAT activity in rats decreased significantly after ischemia injury. These results are consistent with previous reports (29, 32).
SOD is one of the principle enzymatic antioxidants located in cytoplasm and mitochondria and reacts with superoxide radicals to form H2O2. Catalase is implicated to conversion of H2O2 to water and oxygen, leading to diminished toxic effects (33).
As previously mentioned, Withania coagulans has shown different healing properties such as anti-inflammatory, anti-hyperlipidemic, and antioxidant effects (3-6).
As reported by Lateef et al., WCE increases the level of SOD and CAT in hyperlipidemic rabbit (34). Another study demonstrated that Withaniasomnifera elevated the level of SOD and CAT in the striatum of mouse model of Parkinson disease (35).
A recent study showed that WCE increased the total antioxidant capacity (TAC) in benign prostatic hyperplasia in rats (36). These data are in line with the finding of our study indicating that pretreatment with WCE for 30 days improved the mean of SOD and CAT activity in WCE group when compared to I/R group.
The current study also demonstrated that WCE increased GPx activity in striatum region of ischemic pretreated rats. GPx is an important factor for neuroprotection in the brain. Several experiments demonstrated that GPx level decreased in the ischemic brain and treatment with GPx protected neuronal cells from injury (37, 38). Some studies found that consumption of herbal antioxidants could restitute the glutathione level in the brain of ischemia reperfusion in rats (39, 40). Baitharu et al. showed that withanolide-A, derived from Withania, enhances glutathione biosynthesis in neurons and decreases neuronal degradation (41).
The results obtained by Rajasankar et al. revealed that treatment with Withania somnifera increased GPx level in the corpus striatum in the rat model of Parkinson disease that is consistence with our results (35). The striatal cells are vulnerable to oxidative damage and degeneration after ischemia reperfusion. In our study, we found significant histological changes in the striatal neurons in I/R group. The changes observed consisted of demyelination of axon fibers and pycnotic neurons with pericellular space, which is consistent with the previous studies reporting the increase of necrotic neurons in the striatum region and white matter vacuolation (42, 43). In another study, it was shown that WCE protects cortical neurons from degeneration induced by I/R (44).
According to Kuboyama’s study, Withania root extract rich of withanolides has potential effects on neuronal regeneration (45).
The result of our study showed that WCE protects striatal cells from oxidative damage induced by ischemia reperfusion. In the present study, 30 days WCE administration before global ischemia decreased the striatal neuronal damage and demyelination of axon fibers in corpus callosum. Ischemia reperfusion triggers numerous intracellular cascades leading to apoptosis and brain damage. Enhancement of intracellular Ca2+, NF-kB over expression, and up-regulation of pro-inflammatory cytokines, such as TNF-α, following ischemia induce processes leading to neuronal death (46, 47). Several studies confirm that inhibiting the NF-kB signaling pathway could have neuroprotective effects in the model of experimental stroke in rat (48, 49). Some studies have also shown that calcium channel blocking could be effective in attenuating of post ischemic damage induced by I/R (50, 51).
Our results showed that preischemic administration of WCE decreases apoptotic striatal cells and protects neuronal cells in striatum compared to I/R group.
Ali and colleagues have shown that the extract of Withania coagulans has calcium channel blocking activity (52). Moreover, the result of some studies showed that Withania coagulans has a strong anti-inflammatory effect and inhibits up-regulation of pro-inflammatory cytokines, such as TNF-α and IL-1β (53, 54).
On the other hand, Withania extract by enhancement of Bcl-2 protein expression and decrease of Bax protein expression inhibits cell loss in myocardial ischemic reperfusion (55).
Taken together, based on the mentioned studies and our results, WCE by inhibition of TNF-α and NF-κB expression, blocking of calcium ion channels, and reduction of ROS generation has neuroprotective effects against striatal neuron insult induced by I/R.