1. Background
Liver is an important organ that is involved in medicine and xenobiotics metabolism, and is a vital station for substance regulation and bioprocessing (1-3). Liver enzymes neutralize numerous xenobiotics, yet induce bio-activated toxicities, which cause other liver damages (4-7). Metabolism of many drugs, such as acetaminophen, at therapeutic doses is not harmful yet at higher doses oxidative stress may result (8) and subsequently lead to increment of free radicals and reactive oxygen species due to saturation of metabolic pathways. Free radicals are detoxified due to binding to intracellular glutathione and in case of glutathione reduction (3, 9, 10), they will bind to cellular macromolecules, which consequently cause cellular death and liver damage (1, 2). Chronic liver diseases are characterized by progressive evolution from steatosis to chronic hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (2, 11, 12). By changing human lifestyle, liver diseases have become one of the most common and fatal diseases all over the world, particularly in developing countries. Therefore, numerous efforts have been made to explore new drugs of herbal and marine plant origin in order to reduce drug-induced liver injuries (3).
People use algae for centuries because of their high nutritional value. They contain bioactive organic compounds, such as terpenoids and carotenoids. Also, they contain natural antioxidant, sulfated polysaccharides, lipids, proteins, vitamins, and minerals, which are exclusively found in marine plants (2, 13-16)
Sargassum spp. have been shown to have extremely high cleaning property for free radicals (17-21). Herbal polysaccharides are known as a significant group of bioactive natural products (22). Therefore, herbal and marine plants have attracted the attention of pharmaceutical and medical industries due to their demonstrated rich sources of structurally miscellaneous bioactive compounds. Sulfated polysaccharides isolated from seaweeds were investigated to possess immune stimulation (8), anti-tumoral (9, 12, 23), antivirus (10), immunomodulatory (12), and anti-inflammatory properties (11), hepatoprotective (13, 24, 25) and nephroprotective (26) effects of some marine algae have also been reported.
2. Objectives
The aim of this research was to investigate the hampering effects of S. swartzii aqueous extract on acetaminophen-induced hepatotoxicity in mice by virtue of its antioxidant properties.
3. Methods
3.1. Material and Sample Collection
All chemicals at analytical grades were obtained from the Merck company of Germany. Brown algae (Sargassum swartzii) were collected from low tide areas of Boushehr province (Persian Gulf) coasts, during July 2016.
3.2. Preparation of Aqueous Extract of Sargassum swartzii
After transfer to the laboratory, the collected sample was washed with distilled water, air dried, and pulverized in powder. To remove pigments and fat molecules of S. swartzii powder, 100 g of powder was immersed in ethanol (95%). The residue was then extracted from 1:10 volume of distilled water at 90°C for three hours, three times. All water extracts were combined, filtrated, and concentrated in a water bath at 90°C. The residue was stored at 0°C for further analysis (12, 16).
3.3. Animals
Overall, 30 male mice (18 to 27 g) were purchased from the animal house of Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran).
They were kept in standard single cages under standardized conditions (22 ± 4°C). The animals were maintained on standard chow diet and water ad libitum. Furthermore, the Institutional Ethics Committee of the Medicine School of Ahvaz Jundishapur University of Medical Sciences guideline approved all procedures adopted in this study (Ethical number: IR.AJUMS.REC.1396.297).
3.4. Experimental Design
Healthy male mice, weighing 18 to 27g were housed in cages. The animals were kept under standard conditions, seven days after adaptation, and mice were randomly divided to five groups; each of which had six mice. Experimental groups were treated by oral gavage for one week with different doses of aqueous extract of S. swartzii (100 and 200 mg/kg) one hour after receiving acetaminophen (350 mg/kg). Normal saline (5 mL/kg) was orally administered to the negative control group. Also, one hour after receiving acetaminophen (350 mg/kg), the positive control group received normal saline (5 mL/kg), respectively. Finally, the last group received aqueous extract of S. swartzii (100 mg/kg) without acetaminophen administration.
Twenty-four hours after the last administration, animals were anesthetized by Ketamine-Xylazine and serum samples were obtained by heart puncture. Blood collection and centrifugation at 3000 rpm for 15 minutes was performed. Obtained clear supernatant was stored at -70°C prior to analysis for subsequent measurement of ALT, AST, and ALP. Liver was isolated and quickly washed with normal saline, and was then fixed in 10% formalin for histological studies (27-30).
3.5. Serum Biochemical Parameters
The most commonly used markers of liver injury are aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP). Colorimetric determination of ALT and AST were estimated by measuring the amount of pyruvate or oxaloacetate produced by the formation of 2, 4-dinitrophenylhydrazine, according to the method of Reitman and Franker (31); also, colorimetric determination of ALP was estimated by measuring the amount of p-nitrophenyl-phosphate produced by the formation of phosphate substrate when dephosphorylated by ALP (32).
3.6. Histopathology
All mice livers were immediately fixed at 10% formalin for 24 hours, and afterwards embedded in paraffin. Then, they were dehydrated through soaking in alcohol and xylol, respectively. Finally, after preparation of 5-micron tissue sections using rotary microtome, the Haematoxylin and Eosin (H&E) staining technique was performed. The histopathological changes were examined using the light microscope (33).
3.7. Statistical Analysis
Results were presented as mean ± SD values. The statistical significance of differences between groups was determined by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons. A probability value of P < 0.05 was assumed to indicate statistical significance (34).
4. Results
The effects of aqueous extract of S. swartzii on liver marker enzymes are listed in Table 1. These data indicate that the group that received normal saline (NS) showed a normal range of ALT, AST, and ALP levels, whilst the treated group that received acetaminophen showed increased levels of ALT, AST, and ALP, which means acetaminophen (APAP) causes hepatotoxicity at higher dosages.
| Groups | ALT (IU/L) | AST (IU/L) | ALP (IU/L) |
|---|---|---|---|
| NS (A) | 49.33 ± 4.71 | 156.16 ± 9.74 | 354.50 ± 7.66 |
| APAP+NS (B) | 112.16 ± 8.42 | 335.83 ± 8.42 | 698.16 ± 8.72 |
| APAP+S. swartzii 100 mg/kg (f1) | 65.16 ± 7.05 | 184.83 ± 6.56 | 428.66 ± 9.13 |
| APAP+S. swartzii 200 mg/kg (f2) | 55.33 ± 4.76 | 159.33 ± 6.99 | 363.33 ± 4.92 |
| S. swartzii 100 mg/kg + NS (f) | 51.16 ± 5.03 | 157.83 ± 8.28 | 361.16 ± 9.45 |
a Values are expressed as mean ± SD.
The increment of liver marker enzymes are considered as indicators for enzyme release from destroyed cells. On the other hand, the extract-treated group showed interesting results.
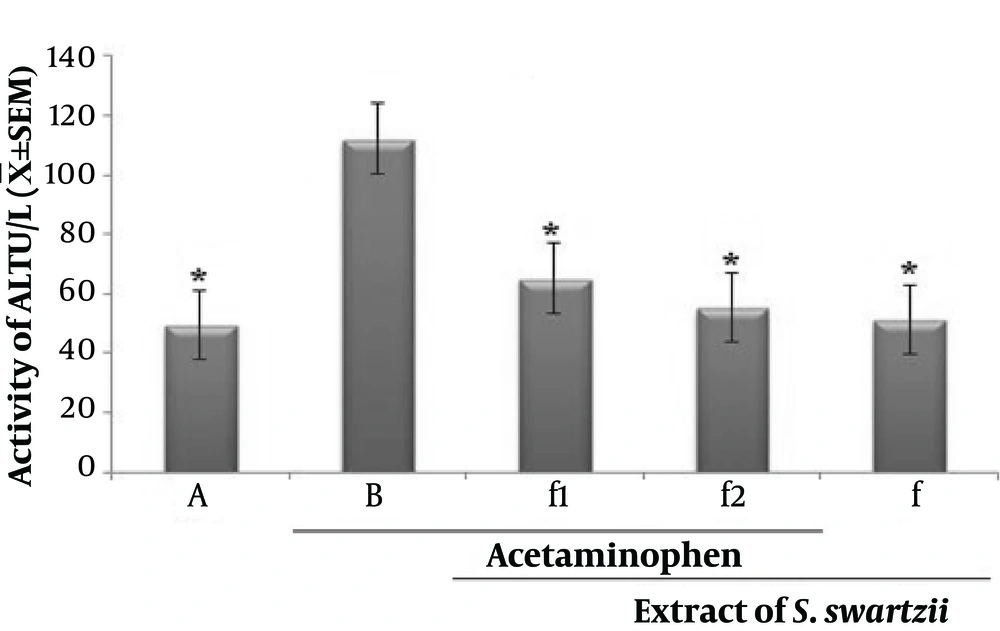
4.1. Effect of Aqueous Extract of S. swartzii on the Serum Levels of ALT
Prescription of acetaminophen (APAP) significantly (P < 0.05) increased the serum levels of ALT when compared to the other groups (*). Oral treatment with S. swartzii (f1, f2) caused a significant decrease in the serum levels of ALT (P < 0.05) in a dose-depended manner. There was a difference between hepatoprotective activity of f1 and f2. Also, no significant (P < 0.05) difference was observed between NS, f, and f2 groups (Figure 1 and Table 1).
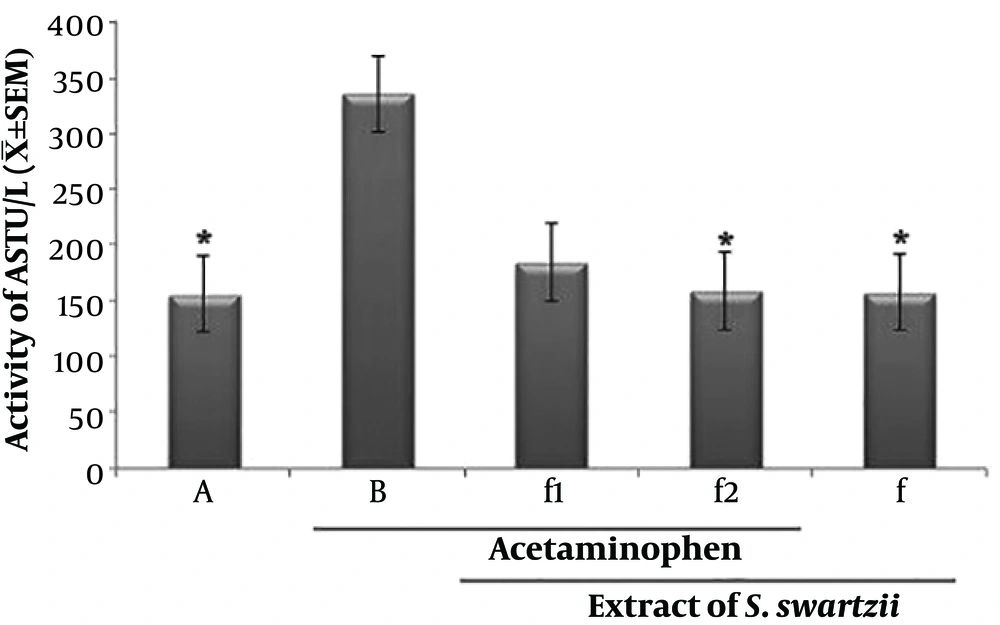
4.2. Effect of Aqueous Extract of S. swartzii on the Serum Levels of AST
The f1 and acetaminophen (APAP) groups significantly (P < 0.05) increased the serum levels of AST when compared to the other groups (*). There was no significant (P < 0.05) difference between NS, f, and f2 groups (Figure 2 and Table 1).
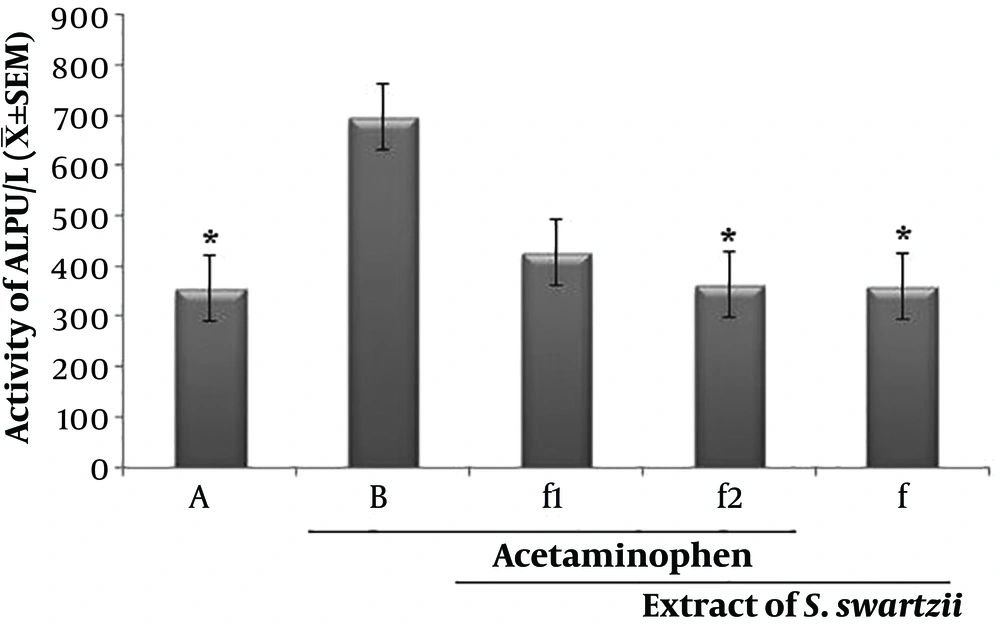
4.3. Effect of Aqueous Extract of S. swartzii on the Serum Levels of ALP
The f1 and Acetaminophen (APAP) groups significantly (P < 0.05) increased the serum levels of ASP when compared to the other group (*). There was no significant (P < 0.05) difference between NS, f, and f2 groups (Figure 3 and Table 1).
Effect of aqueous extract of S. swartzii on the serum levels of ALP. A, group that only received normal saline (NS); B, group that received acetaminophen 350 mg/kg + normal saline; f1, treatment group which received acetaminophen (350 mg/kg) one hour before aqueous extract of S. swartzii (100 mg/kg); f2, treatment group which received acetaminophen (350 mg/kg) one hour before aqueous extract of S. swartzii (200 mg/kg); f, group that only received aqueous extract of S. swartzii (100 mg/kg)
4.4. Histopathology
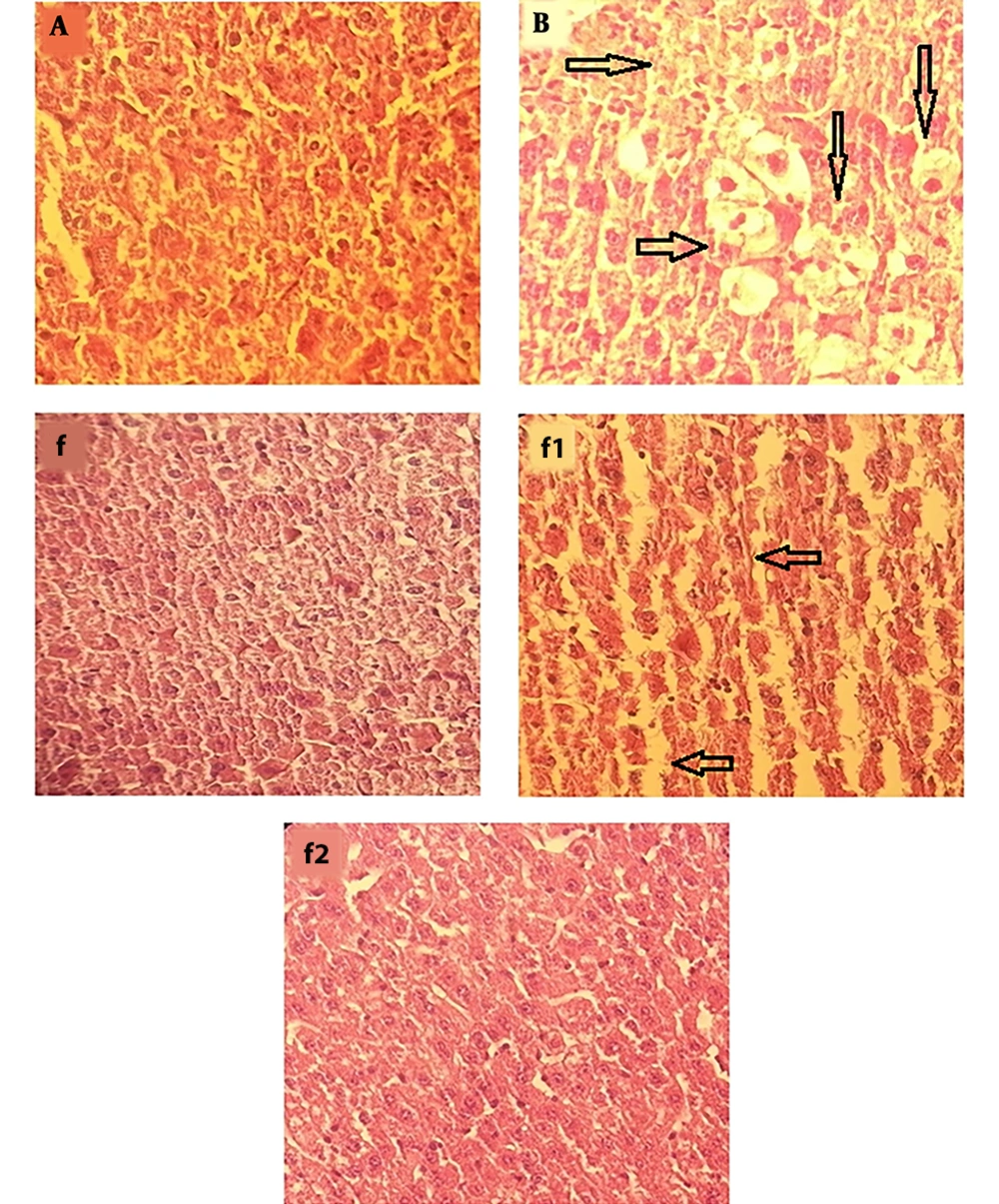
In APAP-treated group (Figure 4B), severe changes were observed in liver morphology, including hepatocytes necrosis, accumulation of inflammatory cells, loss of cellular boundaries, dilated sinusoids, and change in liver cells fat and cell swelling (→) compared with the control group (Figure 4A). Mice that were treated with aqueous extract of S. swartzii (f1 and f2) showed less degeneration of hepatocytes necrosis, reduction of inflammatory cells accumulation, cell swelling in hepatocytes, cellular boundaries, and dilated sinusoids, and these changes in the f2 group showed more complete improvement compared to the f1 group. The group that only received aqueous extract of S. swartzii 100 mg/kg (f) showed a normal structure (groups f, f1, and f2; Figure 4).
Photomicrograph showing histopathological section of liver tissue from each group (original magnification: ×100). A, group that received normal saline (NS); normal morphology of histological section of mice liver; B, group that received acetaminophen 350 mg/kg; f, group that only received aqueous extract of S. swartzii (100 mg/kg); f1, treatment group which received acetaminophen (350 mg/kg) one hour before aqueous extract of S. swartzii (100 mg/kg); f2, treatment group which received acetaminophen (350 mg/kg) one hour before aqueous extract of S. swartzii (200 mg/kg).
5. Discussion
The findings of this study indicate that APAP can induce hepatotoxicity through increasing ALT, AST, and ALP serum levels, which can cause histopathological alterations in liver tissue. Administration of the aqueous extract of S. swartzii (100 and 200 mg/kg) could improve hepatotoxicity induced by APAP via change in liver marker enzymes and also improved histopathological alterations in liver tissue when compared with the APAP group. Thus, it can be suggested that the aqueous extract of S. swartzii (100 and 200 mg/kg) may prevent hepatotoxicity induced by APAP, yet the aqueous extract of S. swartzii (200 mg/kg) significantly (P < 0.05) prevents hepatotoxicity induced by APAP.
In past studies, hepatoprotective effects of different extracts of Sargassum binderi, Sargassum tenerrimum, Sargassum variegatum, and Sargassum polycystum has been investigated (25, 35). According to the obtained results of the present study, aqueous extract of Sargassum swartzii (200 mg/kg) had a more preventive effect on increment of liver enzymes (ALT, AST, and ALP) in comparison to other extracts of Sargassum binderi, Sargassum tenerrimum, Sargassum variegatum, and Sargassum polycystum, which results in better protective effects of Sargassum swartzii (200 mg/kg) on the liver. This is due to the content of their flavonoids, carotenoids, polysaccharides, and phenolic, which can be used as a source of natural non-toxic antioxidants in food, cosmetics, and pharmaceutical industries (2, 20, 36-40).
In the present study, the protective role of the aqueous extract of S. swartzii at different levels was evaluated and confirmed the scientific evidence for pharmacological use of aqueous extract of S. swartzii in liver injuries.