1. Background
Timely diagnosis and treatment of coronavirus disease 2019 (COVID-19) present significant global medical challenges. Physicians face unpredictable clinical courses due to the intricate pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which can rapidly progress to severe and potentially fatal outcomes. Evaluating changes in various biomarkers, including lymphocytes, neutrophils, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), D-dimer, interleukin (IL)-6, creatine kinase (CK), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and coagulation-related markers, is crucial for classifying COVID-19 patients based on disease severity, assessing mortality risk, and selecting appropriate treatments in a timely manner. Coagulation disorders and cytokine-mediated systemic vasculitis play pivotal roles in multi-organ dysfunction and acute respiratory distress syndrome (ARDS) among severe COVID-19 patients (1-3).
To date, the FDA’s EUA has approved several drugs, including monoclonal antibodies (mAbs). Other options, such as traditional medicine, convalescent plasma, neutralizing antibodies (NAbs) treatment (4), and allogeneic cell therapy, are also under investigation. Hydroxychloroquine (HCQ) and chloroquine (CQ), commonly known as anti-malarial and/or antirheumatic drugs, are currently being discussed for their potential anti-SARS-CoV-2 activity. Some studies have demonstrated that HCQ could reduce the viral load in COVID-19 patients, particularly when combined with azithromycin (5, 6). However, the surviving sepsis campaign (SSC) concluded that there is insufficient evidence regarding their effectiveness and safety for routine use in patients undergoing intensive care (7). Certain drug combinations targeting multiple pathways are also under consideration, such as lopinavir plus ritonavir, which is used to treat human immunodeficiency virus (HIV) infection (8). Nevertheless, based on the results from the WHO's solidarity trial involving 11,330 patients across 30 countries, released in December 2020, the HCQ, remdesivir, lopinavir, and interferon regimens showed little or no effect on overall mortality, mechanical ventilation, or duration of hospitalization (8).
Elevated levels of inflammatory cytokines, such as IL-6 and tumor necrosis factor-alpha (TNF-α), have been observed in severe COVID-19 patients compared to moderate cases and healthy individuals. This phenomenon, referred to as "cytokine storm syndrome", is associated with poor prognosis and significant morbidity (9, 10). Drugs targeting inflammatory pathways, such as CQ phosphate, have shown promise in reducing cytokine-mediated damage (11). Similarly, lopinavir/ritonavir, known for its antiviral activity in HIV treatment, was chosen for its potential to inhibit SARS-CoV-2 replication through protease inhibition (12). Ribavirin, a broad-spectrum antiviral agent, was selected due to its historical use against ribonucleic acid (RNA( viruses and its potential synergistic effects with other antiviral agents (13). These drugs were combined to target multiple pathological pathways, including viral replication and excessive inflammatory response, thereby addressing key aspects of severe COVID-19 pathogenesis (14).
2. Objectives
This prospective study aimed to evaluate changes in inflammatory and hematological biomarkers in COVID-19 patients before and after the administration of a combination therapy protocol comprising lopinavir/ritonavir, CQ phosphate, and ribavirin, thereby assessing its effectiveness and safety. The primary goal was to investigate whether this targeted combination therapy could modulate key biomarkers associated with disease severity and adverse outcomes. Overall, this study seeks to bridge the gap between clinical practice and emerging therapeutic strategies, ultimately enhancing the management of COVID-19 patients and potentially improving survival rates.
3. Method
3.1. Study Design and Method
This single-arm prospective study included COVID-19 patients attending the emergency department of Razi Hospital in Ahvaz city, Iran, from 15 September 2020 to 24 October 2020. All procedures involving human participants were conducted in accordance with the ethical standards of the national research committee, the 2008 Helsinki Declaration, and its later comparable ethical standards. Additionally, all procedures were approved by the Ethical Committee of the Research Deputy of Ahvaz Jundishapur University of Medical Sciences under ethical code: IR.AJUMS.REC.1399.001.
Inclusion criteria for this study were COVID-19 patients confirmed by distinct chest computed tomography (CT) scans and positive results of RT-PCR tests, with symptom onset occurring less than seven days prior. Patients were required to be receiving a combination therapy protocol of lopinavir/ritonavir, CQ phosphate, and ribavirin. However, patients who were discharged or transferred to another center before the completion of their clinical or laboratory assessments were excluded from the study. All demographic and clinical data were obtained from the patients’ healthcare records and/or through interviews with their parents by the investigators.
Patients’ clinical and laboratory features were prospectively collected at two time intervals: On the first day of admission (prior to medication initiation) and on the ninth day of treatment. These data were then compared. Laboratory tests were conducted in the hospital laboratory using standard kits (Merck KGaA, Darmstadt, Germany). The levels of several essential laboratory markers, including lymphocyte count, ESR, CRP, LDH, IL-6, and IL-10, were measured at the two time intervals: On the first day of admission before treatment initiation (pre-treatment phase) and on the ninth day of treatment (post-treatment phase). The results were recorded for analysis.
3.2. Doses Used in Combination Therapy
- Lopinavir 400 mg/ritonavir 100 mg tablets: Combined dose of the two medications, administered orally twice daily for 10 - 14 days based on the patient’s clinical status.
- Chloroquine phosphate: 600 mg tablets, administered orally once daily for 4 - 10 days.
- Ribavirin: (1) Oral dose (tablets): 1,000 mg twice daily for 7 - 14 days, depending on clinical response and tolerance; (2) intravenous dose: 200 mg/kg total body weight (divided into doses) administered over 4 - 6 hours daily for 7 - 10 days in severe cases or for patients unable to tolerate oral administration.
Patients receiving these treatments were carefully monitored for adverse effects, particularly liver function (due to lopinavir/ritonavir), cardiac issues (due to CQ), and hematologic complications (due to ribavirin).
3.3. Statistical Analysis
SPSS version 26 statistical software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Categorical variables were compared using the chi-square test and presented as frequencies and percentages, while continuous variables were compared using the paired samples Wilcoxon test and/or the paired t-test, depending on their normality. Additionally, based on the nature of the variables, a multivariate Kruskal-Wallis test and/or multivariable analysis of variance (MANOVA) was employed to compare the differences in biomarker levels at baseline and on the ninth day of treatment. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Characteristics of Patients
Out of a total of 127 studied COVID-19 patients, five were withdrawn from the study due to incomplete laboratory reports. Among the remaining 122 patients, 58 cases (47.5%) had severe COVID-19 infection based on WHO criteria (15). The study population consisted of 69 (56.6%) men and 53 (43.4%) women, with a mean age of 52.81 ± 15.076 years and 52.75 ± 15.70 years, respectively. The prevalence of underlying diseases was estimated at 61.5%. The most common pre-existing comorbidities were diabetes (28%), hypertension (22.7%), and other cardiovascular disorders (20%).
The most prevalent clinical symptoms among COVID-19 patients were fever (84.4%), dry cough (68%), fatigue (64.8%), sputum production (48.4%), muscle or joint pain (48.4%), sore throat (47.5%), and headache (49.2%). The mean length of hospital stay was 6.9 ± 3.7 days. The survival rate was 94.3%, with 7 patient fatalities recorded (Table 1).
| Variables | Values a |
|---|---|
| Age, y | |
| < 40 | 23 (18.9) |
| 41 - 50 | 37 (30.2) |
| 51 - 60 | 28 (23) |
| > 60 | 34 (27.9) |
| Gender | |
| Male | 69 (56.6) |
| Female | 53 (43.4) |
| Clinical signs | |
| Fever | 103 (84.4) |
| Dry cough | 83 (68) |
| Fatigue | 79 (64.8) |
| Sputum production | 59 (48.4) |
| Muscle or joint pain | 59 (48.4) |
| Sore throat | 58 (47.5) |
| Headache | 60 (49.2) |
| Diarrhea | 4 (3.3) |
| Underlying diseases | 75 (61.5) |
| Infection severity | |
| Non severe | 64 (52.5) |
| Severe | 58 (47.5) |
| Length of hospitalization (1 - 19 days) | 6.8 ± 3.7 |
| Survival status | |
| Improved or recovered | 115 (94.3) |
| Dead | 7 (5.7) |
Demographic and Clinical Characteristics of All Coronavirus Disease 2019 Patients
4.2. Comparison of Laboratory Features in Patients Before and After Treatment
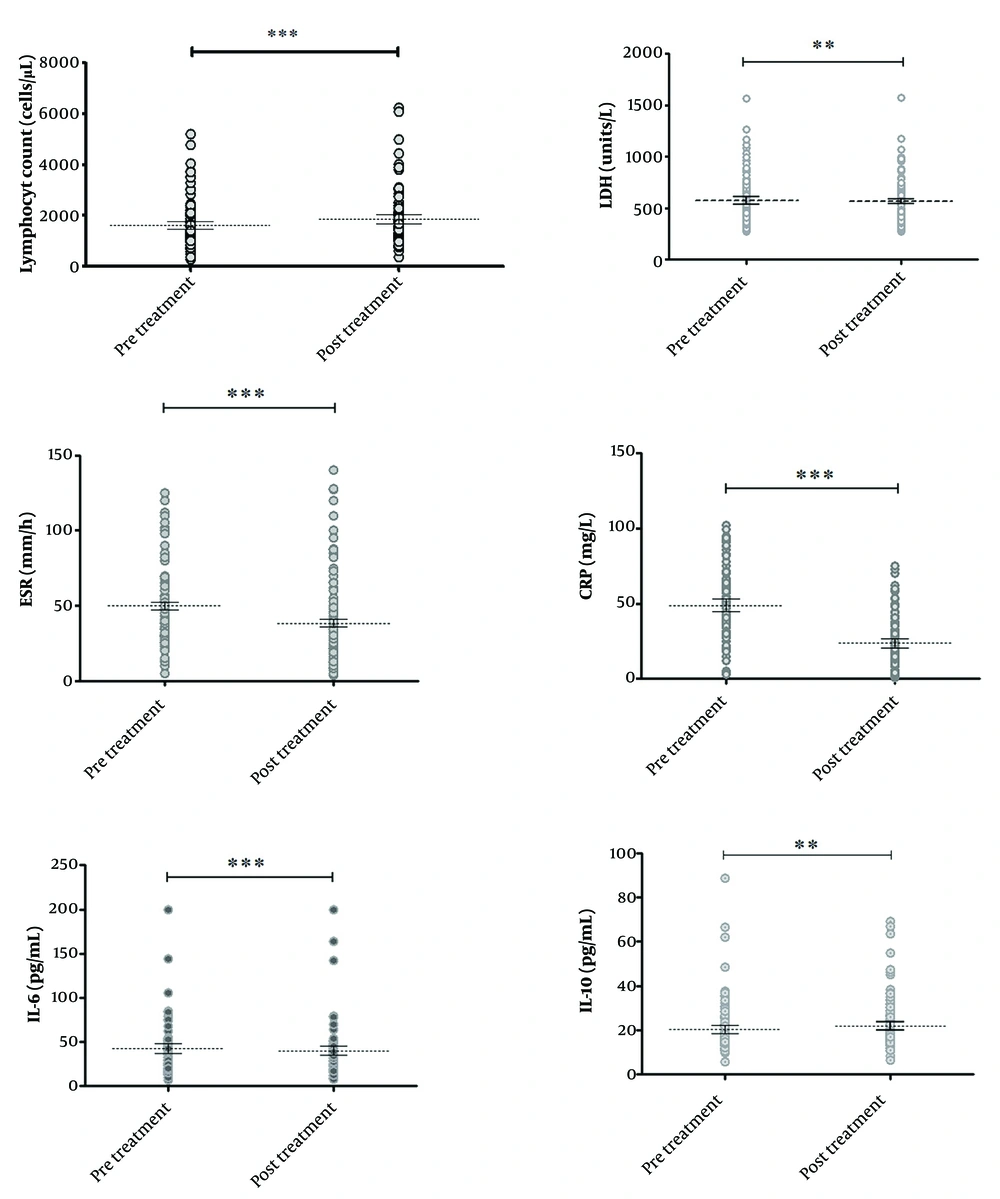
As shown in Table 2, the mean levels of lymphocytes and IL-10 on the ninth day of treatment (post-treatment phase) were significantly higher than their levels on the first day of admission (pre-treatment phase, or prior to medication initiation) (P < 0.05). Additionally, the mean levels of LDH, IL-6, ESR, and CRP were significantly decreased after treatment (P < 0.05), as illustrated in Figure 1.
| Laboratory Markers | N | Pre-treatment Phase | Post-treatment Phase | Mean of Differences | P-Value a (t-Test) | P-Value a (MANOVA) |
|---|---|---|---|---|---|---|
| Lymphocyte (cells/µL) | ||||||
| Total | - | 1624 ± 834.8 | 1852 ± 964.1 | -216.1 | 0.0002 | |
| Female | - | 1875 ± 930.4 | 2130 ± 1028 | -255.1 | 0.004 | |
| Male | - | 1431.58 ± 701 | 1632.18 ± 855.7 | -185.2 | 0.01 | |
| P-value (paired t-test) | - | 0.008 | 0.02 | 0.7 | ||
| LDH (units/L) | ||||||
| Total | - | 574.52 ± 224.7 | 566.7 ± 221.32 | 7.82 | 0.008 | |
| Female | - | 557.5 ± 212.45 | 551 ± 201.5 | 6.47 | 0.16 | |
| Male | - | 607.82 ± 236.97 | 601 ± 243 | 6.8 | 0.02 | |
| P-value (paired t-test) | - | 0.18 | 0.17 | 0.89 | ||
| IL-6 (pg/mL) | ||||||
| Total | - | 42.81 ± 31.68 | 40.07 ± 29.35 | 2.73 | 0.0002 | |
| Female | - | 43.88 ± 35.655 | 40.32 ± 31.97 | 3.56 | 0.004 | |
| Male | - | 42 ± 28.5 | 39.88 ± 27.42 | 2.1 | 0.01 | |
| P-value (paired t-test) | - | 0.64 | 0.66 | 0.83 | ||
| IL-10 (pg/mL) | ||||||
| Total | - | 20.36 ± 11.09 | 21.9 ± 10.97 | -1.53 | 0.006 | |
| Female | - | 20.05 ± 11.23 | 22.03 ± 10.06 | -1.98 | 0.01 | |
| Male | - | 20.597 ± 11.0452 | 21.7768 ± 11.690 | -1.18 | 0.13 | |
| P-value (paired t-test) | - | 0.6 | 0.9 | 0.46 | ||
| ESR (mm/h) | ||||||
| Total | - | 49.87 ± 27.85 | 38.38 ± 29.97 | 10.73 | 0.0001 | |
| Female | - | 51.94 ± 28.6 | 39.4 ± 30.1 | 11.42 | 0.006 | |
| Male | - | 48.28 ± 27.36 | 37.58 ± 30.08 | 10.19 | 0.0004 | |
| P-value (paired t-test) | - | 0.13 | 0.03 | 0.5 | ||
| CRP | ||||||
| Total | - | 48.90 ± 22.41 | 23.70 ± 16.64 | 24.74 | 0.0001 | |
| Female | - | 49.71 ± 24.70 | 24.22 ± 16.62 | 25.48 | 0.0001 | |
| Male | - | 48.28 ± 20.64 | 23.28 ± 16.78 | 24.15 | 0.0001 | |
| P-value (paired t-test) | - | 0.55 | 0.5 | 0.9 | ||
| Lymphocyte | 0.45 | |||||
| < 40 b | 23 | 1676 ± 872.5 | 1998 ± 1094 | 321.96 ± 699.4 | ||
| 41 - 50 b | 28 | 1690 ± 921.9 | 2018 ± 1195 | 318.07 ± 725.82 | ||
| 51 - 60 b | 34 | 1695 ± 945.8 | 1844 ± 973.1 | 149.38 ± 443.45 | ||
| 61 - 90 b | 35 | 1510 ±625 | 1631 ± 581.8 | 121.31 ± 609.28 | ||
| P-value (MANOVA) | - | 0.77 | 0.36 | |||
| LDH | 0.13 | |||||
| < 40 b | 23 | 566.5 ± 268.7 | 567.7 ± 268.1 | 1.26 ± 13.27 | ||
| 41 - 50 b | 27 | 604.7 ± 216.5 | 588 ± 192.3 | -7.26 ± 17.56 | ||
| 51 - 60 b | 34 | 502.1 ± 171.6 | 496.1 ± 175.4 | -5.97 ± 15.12 | ||
| 61 - 90 b | 35 | 623.3 ± 235.1 | 614.8 ± 239.5 | -11.65 ± 28.44 | ||
| P-value (MANOVA) | - | 0.12 | 0.14 | |||
| IL-6 | 0.55 | |||||
| < 40 b | 23 | 41.15 ± 36.55 | 39.75 ± 29.48 | -1.4 ± 8.38 | ||
| 41 - 50 b | 27 | 41 ± 19.28 | 36.99 ± 16.12 | -3.98 ± 9.19 | ||
| 51 - 60 b | 34 | 36.86 ± 18.18 | 34.96 ± 15.26 | -1.89 ± 6.41 | ||
| 61 - 90 b | 35 | 50.69 ± 43.28 | 47.31 ± 43.26 | -3.38 ± 6.66 | ||
| P-value (MANOVA) | - | 0.3 | 0.3 | |||
| IL-10 | 0.93 | |||||
| < 40 b | 23 | 20.65 ± 16.06 | 21.60 ± 13.47 | 0.95 ± 6.93 | ||
| 41 - 50 b | 27 | 18.52 ± 7.18 | 20.46 ± 7.437 | 1.84 ± 2.9 | ||
| 51 - 60 b | 34 | 20.23 ± 9.87 | 22.09 ± 9.55 | 1.86 ± 3.79 | ||
| 61 - 90 b | 35 | 21.69 ± 11.09 | 22.96 ± 12.87 | 1.71 ±7.2 | ||
| P-value (MANOVA) | - | 0.72 | 0.84 | |||
| ESR | 0.27 | |||||
| < 40 b | 23 | 42.09 ± 22.83 | 40.39 ± 22.96 | -1.69 ± 22.07 | ||
| 41 - 50 b | 27 | 53.46 ± 30.1 | 38.56 ± 27.63 | -12.81 ± 19.84 | ||
| 51 - 60 b | 34 | 50.44 ± 31.04 | 39.21 ± 37.37 | -11.23 ± 29 | ||
| 61 - 90 b | 35 | 51.46 ± 25.95 | 36.11 ± 28.8 | -14.57 ± 26.76 | ||
| P-value (MANOVA) | - | 0.5 | 0.95 | |||
| CRP | 0.03 | |||||
| < 40 b | 23 | 40.78 ± 17.17 | 28.08 ± 16.19 | -12.7 ± 20.06 | ||
| 41 - 50 b | 27 | 47.25 ± 24.38 | 20.86 ± 16.97 | -24.66 ± 22.07 | ||
| 51 - 60 b | 34 | 51.08 ± 23.67 | 21.85 ± 13.65 | -29.23 ± 22.3 | ||
| 61 - 90 b | 35 | 53.17 ± 21.9 | 24.87 ± 19.12 | -26.97 ± 20.27 | ||
| P-value (MANOVA) | - | 0.18 | 0.4 |
Comparison of Biomarker Changes Between Pre-treatment and Post-treatment Phase in COVID-19 Patients, with Additional Stratification by Gender and Age Groups
Comparison of the levels of biomarkers in coronavirus disease 2019 (COVID-19) patients between the two phases: The first day of admission (pre-treatment phase, prior to medication initiation) and the 9th day of treatment (post-treatment phase). ** P-value < 0.01 and *** P-value < 0.001 is considered statistically significant.
A comparison of changes in biomarker levels based on gender showed no significant differences in pre-treatment levels between the two genders, except for lymphocyte count, which was significantly lower in males. However, the decrease in LDH levels was more evident in men, while the increase in IL-10 levels was more pronounced in women (Table 2).
Based on a MANOVA, only the level of CRP differed significantly among various age groups (P = 0.03). The change in CRP levels was significantly more pronounced in patients over 50 years of age compared to other groups (Table 2).
Furthermore, no correlation was found between disease severity and sex (P = 0.85) or age range (P = 0.47). Similarly, no correlation was observed between sex and the presence of underlying disease (P = 0.19). Nevertheless, disease severity was significantly associated with the presence of an underlying disease [P = 0.005, OR (95% CI): 2.96 (1.39 - 6.31)].
5. Discussion
This study evaluated changes in COVID-19-associated biomarkers across two phases: The pre-treatment phase (prior to medication initiation) and the post-treatment phase (on the ninth day of administering a combination therapy protocol comprising lopinavir/ritonavir, CQ phosphate, and ribavirin). The primary objective was to assess the effectiveness and safety of this treatment regimen in COVID-19 patients.
The findings revealed that the levels of lymphocytes, LDH, IL-6, IL-10, ESR, and CRP were significantly modulated after treatment, as observed on the ninth day of therapy compared to baseline levels. However, with a mortality rate of 5.7%, questions remain regarding the safety of this treatment protocol for COVID-19 patients.
Following the publication of the 2020 WHO Solidarity Trial (8), the WHO issued strong recommendations against the use of HCQ and lopinavir/ritonavir, as these drugs showed no significant impact on overall mortality, the initiation of ventilation, or the duration of hospitalization.
Various biomarkers, particularly inflammatory markers, play a key role in the early diagnosis, timely treatment, and prognosis of COVID-19. Several studies have identified that elevated levels of CRP, IL-6, liver and kidney-related markers (AST, ALT), creatinine, blood urea nitrogen, white blood cell count, and low levels of lymphocytes, platelets, and albumin are directly associated with severe COVID-19 infection and an increased mortality risk (16-18). SARS-CoV-2 induces the high-level secretion of IL-6, IL-10, IL-2, IL-4, TNF-α, interferon-gamma (IFN-γ), CRP, IL-8, monocyte chemoattractant protein-1 (MCP-1), IFN-γ -induced protein 10 (IP-10), macrophage inflammatory protein-1 alpha (MIP-1a), macrophage inflammatory protein-1 beta (MIP-1b), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and regulated on activation, normal T-cell expressed and secreted (RANTES), especially in severe cases (10, 19).
Several drugs targeting different COVID-19 biomarkers are under consideration, including mAbs [Kevzara, Leronlimab, LY-CoV555, REGN-COV2, CERC-002, and S309 targeting the IL-6 receptor, CCR5, S-protein, and LIGHT cytokine, respectively], recombinant antibodies [47D11 and AbEpic (against S-protein), APN01 targeting ACE2], small-molecule compounds [remdesivir, HCQ and CQ, dexamethasone, and others], allogeneic cell therapy (CAP-1002), convalescent serum/plasma (passive immunity), and traditional medicine (20). However, their therapeutic effects for COVID-19 are still debated.
Th1-type activation leads to the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), while T helper 2 (TH2) cell activation induces the release of anti-inflammatory cytokines (IL-4 and IL-10) (21). The levels of T helper cells (CD4+) and suppressor T-cells were abnormally low in COVID-19 patients, and lower levels of T helper cells were associated with severe infection. Natural killer cells and cytotoxic lymphocytes play a vital role in controlling viral infections. Abnormal levels of lymphocyte counts in severe COVID-19 reflect a low level of CD4+ cells and memory helper T-cells, as well as an increase in naive helper T-cells. The total counts of T-cells, B-cells, CD8+ T-cells, and natural killer cells were notably decreased in COVID-19 patients, particularly in severe infections (20, 22-24).
Furthermore, the present study did not find any association between gender and/or age with changes in biomarkers, except for CRP, which was significantly more reduced in the age range > 50 years compared to the age range < 40 years. Additionally, current evidence shows that the presence of an underlying disease in patients increases the severity of the infection. However, no correlation was found between the severity of the disease and sex or age range.
Unlike retrospective studies, prospective studies are more useful for accurately identifying causal relationships between the type of therapeutic intervention and the outcomes. However, the inability to adjust the study in the form of a randomized clinical trial may reduce the accuracy of our results.
5.1. Conclusions
A combination therapy protocol of lopinavir/ritonavir, CQ phosphate, and ribavirin may exert a significant ameliorative effect on the inflammatory and hematological parameters associated with COVID-19. However, definitive conclusions regarding its impact on clinical outcomes require further investigation.