1. Background
The ongoing global pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has created numerous challenges worldwide (1). Physicians are confronted with an unpredictable clinical course due to the complex pathophysiology of SARS-CoV-2, which can rapidly progress to severe and potentially fatal outcomes. Consequently, timely treatment of COVID-19 has become a global medical challenge (1). Numerous clinical trials have been conducted to identify effective treatments for COVID-19 patients (2), including antiviral drugs such as lopinavir-ritonavir, remdesivir, favipiravir, arbidol, ribavirin, chloroquine (CQ), and hydroxychloroquine (HCQ); antibiotics; immune modulators such as convalescent plasma (3), interleukin inhibitors, baricitinib, corticosteroids, anakinra (Kineret®), tocilizumab; and anticoagulants (3, 4). Although many therapeutic options have shown partial efficacy in improving COVID-19 patient recovery, the lack of sufficient evidence regarding their definitive effects and the presence of side effects cannot be overlooked. Notable side effects include: (A) An increased risk of bradycardia with lopinavir-ritonavir during critical stages of infection (5); (B) nausea and respiratory issues in patients undergoing prolonged remdesivir treatment (6); (C) anemia development, particularly in severe cases, following baricitinib treatment (7); (D) immunosuppression associated with corticosteroids and anakinra (8, 9); and (E) anemia and elevated alanine aminotransferase levels during tocilizumab treatment (10). Further research is necessary to discover definitive treatments with minimal side effects. In this context, herbal medicine has been considered for COVID-19 treatment (11). Remdesivir (GS-5734) has demonstrated in vitro activity against SARS-CoV-1 and Middle East respiratory syndrome (MERS-CoV) and may prevent COVID-19 progression (12). Additionally, drug combinations targeting multiple pathways, such as Actemra® (tocilizumab) with remdesivir, are under consideration (13). However, the WHO’s solidarity trial across 30 countries indicated that remdesivir has no significant effect on mortality or other critical outcomes in COVID-19 patients (14).
2. Objectives
The present single-arm, non-randomized clinical trial aims to assess the therapeutic effect of remdesivir on COVID-19 patients.
3. Methods
3.1. Study Design
This single-arm, non-randomized clinical trial was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, with ethical code: (IR.AJUMS.REC.1399.407) and IRCT20200404046937N5. Eligible patients who presented to the Infection Departments of Razi Teaching Hospital in Khuzestan province, Iran, were enrolled in this study. The trial included at least a one-week treatment period and an eight-week follow-up period. The local institutional ethics committee of the study center oversaw the proceedings and documentation.
3.2. Patients
Eligible voluntary patients (aged ≥ 18 years) who exhibited specified symptoms of COVID-19 and tested positive for SARS-CoV-2 via RT-PCR and CT scan were included in this trial. Patients with the following conditions were excluded from the study:
(A) Pregnant and lactating women
(B) Patients with other acute diseases, such as malignancies, advanced heart failure, liver cirrhosis, stroke, Alzheimer’s disease, advanced neurological diseases, advanced respiratory disease, and those undergoing dialysis
(C) Individuals with hypersensitivity to remdesivir
(D) Patients who received other antiviral drugs except those mentioned in the national protocol
(E) Patients with multiple organ failure
(F) Patients in a serious condition under mechanical ventilation at the beginning of the study
(G) Patients with alanine transaminase (ALT) and aspartate transaminase (AST) levels up to 5 times the normal limit
(H) Patients with creatinine clearance less than 50 mL/min
3.3. Intervention
Patients received intravenous remdesivir at a dose of 200 mg on the first day, followed by 100 mg on the next four days, administered as single daily infusions over five days.
3.4. Outcomes
The primary outcomes were the length of in-hospital stay (LOS) and the duration of the recovery period of COVID-19 symptoms. Secondary outcomes included the attendance rate in the intensive care unit (ICU), two-month mortality post-admission, and the frequency of remdesivir-related side effects.
3.5. Statistical Analysis
In this study, continuous variables were reported as mean and standard deviation (SD). Categorical variables were reported as numbers and percentages. The Friedman test was used to analyze the time effect on categorical outcome variables. Data were analyzed using SPSS software version 26 (SPSS Inc., Chicago, IL, USA), and P-values < 0.05 were considered statistically significant. Plots were displayed using GraphPad Prism 6.
4. Results
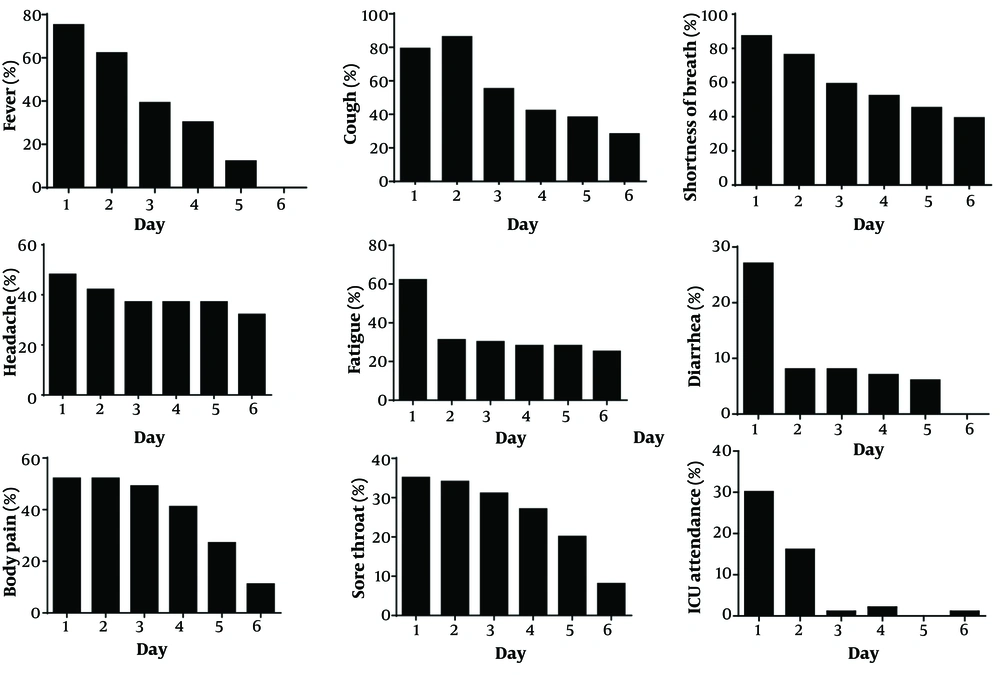
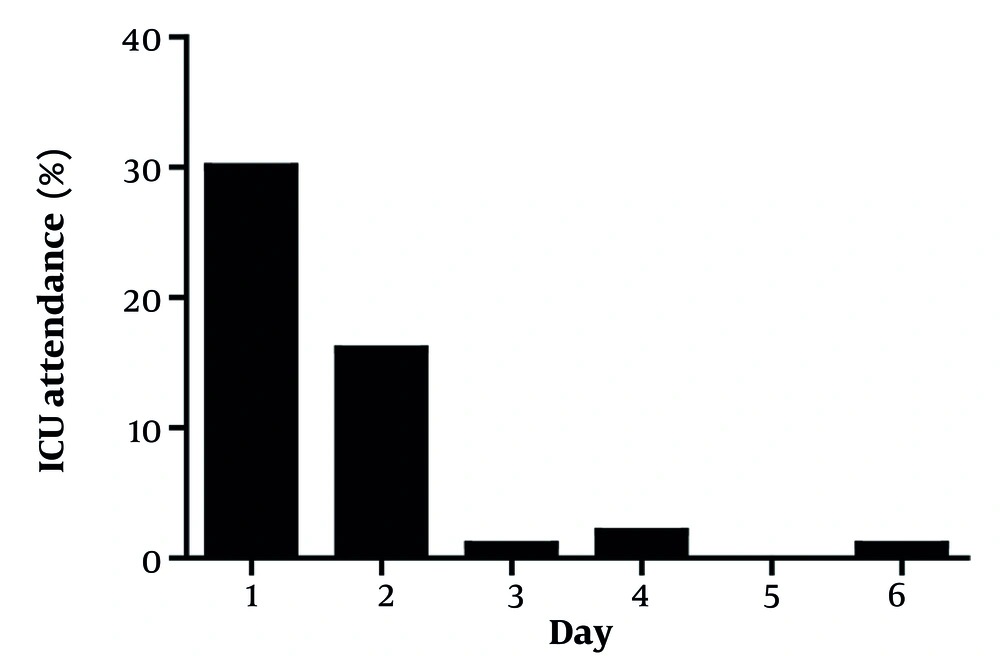
A total of 71 eligible COVID-19 patients were enrolled. Patients were more frequently male (63.4% vs. 36.6% female). Most patients had underlying diseases (60.6%) (Table 1). Overall, the mean ± SD LOS was 6.55 ± 3.81 days, and most patients (59.15%) recovered and were discharged in less than 7 days (Table 1). As shown in Figure 1, the trend of recovery time was significant (P < 0.001) for all symptoms based on the Friedman test. Specifically, symptoms such as fever and diarrhea improved in less than 6 days. Additionally, as shown in Figure 2, a significant decreasing trend was observed in the rate of ICU attendance (P < 0.001). Ultimately, 14 patients (19.72%) died, and no side effects were observed in patients.
| Variables | No. (%) or Mean ± SD |
|---|---|
| Age | 55.48 ± 14.09 (27 - 94) |
| LOS (d) | 6.55 ± 3.81 (1 - 20) |
| < 7 | 42 (59.15) |
| ≥ 7 | 29 (40.85) |
| Gender | |
| Male | 45 (63.4) |
| Female | 26 (36.6) |
| Underlying disease | 43 (60.6) |
| Died | 14 (19.72) |
| Alive | 57 (80.28) |
Demographic and Clinical Data of Coronavirus Disease 2019 Patients Underwent Remdesivir Treatment
5. Discussion
Remdesivir (GS-5734) has demonstrated in vitro activity against SARS-CoV-1 and the Middle East respiratory syndrome. Its active form can inhibit the RNA-dependent RNA polymerase (RdRp) of coronaviruses such as SARS-CoV-2 (15). To date, various clinical evidence has confirmed the significant antiviral effect of remdesivir in improving COVID-19 patients (16). However, according to WHO trial reports, there is no definitive clinical evidence confirming the therapeutic effect of this drug, and remdesivir has little or no effect on survival (14). Our findings showed a trend of recovery time for all symptoms in COVID-19 patients who underwent one week of treatment with remdesivir. Additionally, most patients recovered in less than 7 days. In contrast, findings from a randomized, double-blind, placebo-controlled, multicenter trial conducted by Wang et al. showed no association between remdesivir use and faster time to clinical improvement compared to those receiving placebo [hazard ratio 1.52 (0.95 - 2.43)]. They reported a notable rate of adverse events in remdesivir recipients (66%) versus 64% of 78 placebo recipients (17). However, no adverse events were observed in remdesivir recipients in our trial. The higher mortality rate in our study population (19.72%) compared to their study’s remdesivir recipients (10%) indicates a lack of significant effect of remdesivir on the mortality rate in COVID-19 patients. Given that Wang et al.’s trial investigated only patients with severe COVID-19, our contradictory results regarding remdesivir’s effect on recovery time may be due to differences in disease severity between the two study populations, as a significant percentage of our remdesivir recipients had mild to moderate COVID-19 infection. In our study, most patients were male and had underlying diseases, suggesting that men, particularly those with underlying conditions, are at higher risk for COVID-19 infection. This finding aligns with other similar COVID-19 studies (18, 19). Gottlieb et al. evaluated the effect of early prescribing of remdesivir on preventing the progression of COVID-19 to severe infection in outpatients through a randomized, double-blind, placebo-controlled trial. They reported that a 3-day course of remdesivir showed an acceptable safety profile and led to an 87% lower risk of death than placebo (18). Although our results confirmed the safety of remdesivir, its effect on reducing the mortality rate was not notable. Based on a randomized, open-label, phase 3 trial conducted by Spinner et al. on 584 patients with moderate COVID-19, patients who underwent a 5-day course of remdesivir showed significantly better clinical status at 11 days after treatment initiation compared with those who received standard care (19). However, WHO released a conditional recommendation against the use of remdesivir in COVID-19 patients on November 20, 2020, stating that regardless of disease severity, there is currently no evidence on the effectiveness of remdesivir in improving clinical outcomes and survival (https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-19-patients). Mortality and other outcomes may be strongly influenced by clinical status and infection severity in hospitalized COVID-19 patients (12). Another WHO solidarity trial conducted on 11,330 COVID-19 inpatients across 40 countries reported that HCQ, remdesivir, lopinavir, and interferon regimens had little or no significant effect on mechanical ventilation status, mortality, and LOS (20). Our findings indicated a significant reduction trend for the rate of ICU attendance in COVID-19 patients. However, despite the prescription of remdesivir, the mortality rate among our patients was notable (19.72%), confirming the WHO report about no significant effect of remdesivir on the mortality rate.
5.1. Conclusions
Our findings indicate a significant reduction trend for the recovery time of all symptoms as well as the rate of ICU attendance in COVID-19 patients who underwent 5 days of treatment with remdesivir. Additionally, male gender and underlying disease may be potential risk factors associated with COVID-19 infection. Most patients treated with remdesivir recovered in less than 7 days. However, the mortality rate was not significantly improved by remdesivir. Adding a randomized control/placebo group to the single-arm trial is recommended to improve the reliability of results.