1. Background
Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs, also known as chemotherapeutic agents or alkylating agents (1). Methotrexate (MTX) is a chemotherapeutic and immunosuppressive agent (2). The mechanism of MTX’s effect on men’s fertility and the pregnancy outcomes of their wives is due to its impact on the dividing cells of testicular tissue, resulting in damage or death. This process occurs continuously during sperm production (3). There are many reports about the reversible sterility of men who have used MTX (4, 5), which is indirectly related to the state of testicular tissue. In them, it is mentioned that the quality of sperm decreases, and after stopping the drug, the sperm returns to the normal level and quality (6). Oxidative stress is related to an increase in the production of oxidizing species or a significant decrease in the effectiveness of antioxidant defense (7). Oxidative stress is caused by an increase in the production of oxidizing species or a significant decrease in the effectiveness of antioxidant defense (8). Thiamine (vitamin B1) is an essential micronutrient for humans and animals, belonging to the water-soluble vitamin family (9). This vitamin plays a role in the body both in its free form and as phosphate esters, such as thiamine monophosphate (TMP), thiamine pyrophosphate (TPP), and thiamine triphosphate (TDP) (10). Thiamine pyrophosphate also helps improve the level of antioxidants by participating in the pentose phosphate pathway and aiding in NADPH production (11). Studies show that thiamine therapy should be an important part of treatment plans and health policies to prevent the progression of some metabolic diseases (12).
2. Objectives
Given the confirmation of the participation of this factor in the spermatogenesis process and the antioxidant properties of thiamine, the role of this vitamin in treating and compensating for testicular tissue damage in male infertility is conceivable.
3. Methods
3.1. Animal Models
This experiment was conducted on 36 adult and healthy Balb/c male mice weighing 25 - 30 grams. The treatment was carried out for four weeks under standard conditions including temperature of 23.00 ± 3.00°C and 12-hour light/dark cycle, 30 - 60% humidity and day and night lighting cycles. All the procedures performed on the animals were carried out in accordance with the guidelines of the Ethics Committee (IR-UU-AEG-3/63) of the Faculty of Veterinary Medicine of Urmia University.
Mice were randomly divided into 6 groups of 5 pieces as follows: In healthy control group 1, only 0.1 mL normal saline was injected intraperitoneally (IP), group 2 (MTX group) was injected MTX 10 mg/kg/week, IP (13). Group 3 (positive control group) was injected VitB1 100 mg/kg, IP. Groups 4, 5 and 6, received MTX + VitB1 with doses of 25, 50, 100 mg/kg/day, IP, respectively. As the present study aimed to evaluate the protective effect of thiamine, this vitamin was used in three double doses based on previous works (14).
Vitamin B1 from Rooyan Darou Co. (Tehran, Iran) and MTX sodium manufactured Kocak Farma Ltd. (Istanbul, Turkey) were used as the test materials.
3.2. Necropsy and Tissue Sampling
After 36 days of treatment, for histological examination, animals were euthanized with overdose of ketamine (100 mg/kg, Alfasan, Voorden, the Netherlands) and xylazine (5.00 mg/kg, Alfasan). Their right testes were taken for histomorphometrical analysis and left testicles were sampled and stored at -70°C to detect the activity of MDA, TAC and caspase 3&7 in the testis tissue.
3.3. Histological Analysis
The right testes were fixed by 10% formaldehyde. Then paraffin sections were prepared by digital microtome (Microm, GMBH, and Germany) and transferred to slides. Slides were stained with hematoxylin and eosin (H&E) method (15). Histomorphometric studies including the measurement of the thickness of the germinal epithelium, the diameter of the testicular capsule, the diameter of the seminiferous tubules, the counting of the spermatogenic cell layers, primary spermatocytes, Leydig cells and active Sertoli cells were performed. Also, seminiferous tubules with more than 3 germinal layers counted as positive Tubular Differentiation Index (TDI), tubules with growing sperm counted as tubules with a Positive Spermiogenesis Index (SPI) and the tubules with high percentages of spermatogonia type B (dark nucleus) relative to spermatogonia type A (light nucleus) were considered as tubules with positive Repopulation Index (RI) in 10 cross-sections from the each group and the results were compared between the groups which evaluated using a light microscope (Olympus, Tokyo, Japan) with calibrated graded optical lens (16). Qualitative assessment of fertility and the spermatogenesis process according to Johnson’s scoring scale:
(1) Germ and Sertoli cells cannot be seen. Tubules are atrophic.
(2) There are no germ cells, only Sertoli cells can be seen.
(3) There are no primary spermatocytes. Just spermatogonia can been seen.
(4) Very few primary spermatocytes can be seen.
(5) There is no sperm and round spermatid. A large number of primary spermatocytes can be seen.
(6) A few round spermatids can be seen.
(7) There is no sperm; however, a large number of round spermatids are visible.
(8) Sperm count is very low.
(9) There is a large number of sperm but round sperm cannot be seen and the lumen has no regular contour.
(10) Full spermatogenesis, lots of sperm that are regularly rounded in the edge of the lumen (17).
3.4. Histochemical Examinations of Testicular Tissue
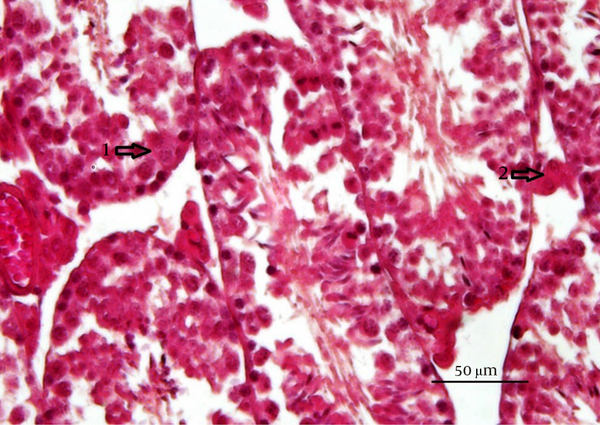
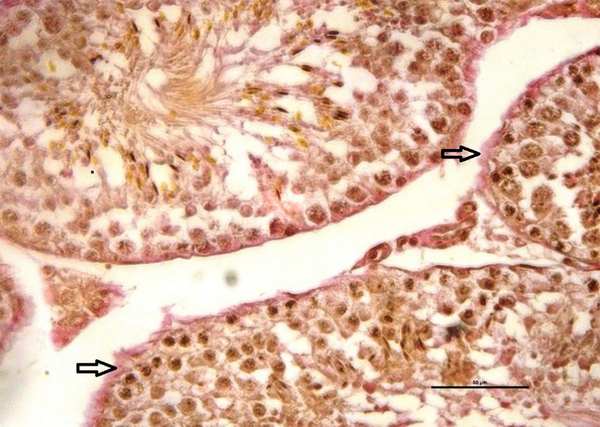
Van Gieson’s staining method was performed for study of distribution of collagen (18). Toluidine blue staining method performed for detecting of the mast cells. The granules of these cells stained as buleshred or metachromasia (different color from the dye solution) (19). Periodic acid-Schiff (PAS) staining method, used to detect carbohydrate compound substances in testicular tissues (15). All image data were analyzed by software (NIKON, ECLIPSE, Ts2R, NIS-Elements, AR4.60.00).
3.5. Evaluation of Malondialdehyde
To determine the extent of lipid peroxidation, the MDA content of the collected testis samples was measured using the thiobarbituric acid (TBA) reaction (20). Briefly, 0.3 - 0.4 g of testicular tissue was homogenized in ice-cold KCl (150 mM), and then the mixture was centrifuged at 3000 × g for 10 min. After that, 0.5 mL of the supernatant was mixed with 3 mL of 1% phosphoric acid and after vortex mixing, 2 mL of 6.7 g/L TBA was added to the samples (21).
3.6. Evaluation of Total Antioxidant Capacity
The evaluation of total antioxidant capacity (TAC) was performed using the iron reduction antioxidant power (FRAP) assay. Briefly, at acidic pH, which was created using acetate buffer (300 mM, pH 3.6), the reduction of FeIII-TPTZ (2, 4, 6-tri-2-pyridyl-1, 3, 5-triazin, Merck, Germany) complexed to iron form, deep blue color was measured at 593 nm (22).
3.7. Caspase 3&7 Activity
Caspase 3&7 activity was measured in testicular tissue samples using a colorimetric caspase 3&7 assay kit (Kiasyst, Hamadan). This assay is based on monitoring the cleavage of DEVD-p-nitroanilide in to yellow p-nitroaniline (pNA) over time (23).
3.8. Statistical Analysis
Statistical analyses were performed using SPSS (version 21, USA). The quantitative histomorphometric, biochemical, and in-vitro fertilization data were compared between all groups and analyzed using one-way ANOVA, followed by Bonferroni post-hoc test. P-value < 0.05 was considered significant. All data were presented as mean ± SE.
4. Results
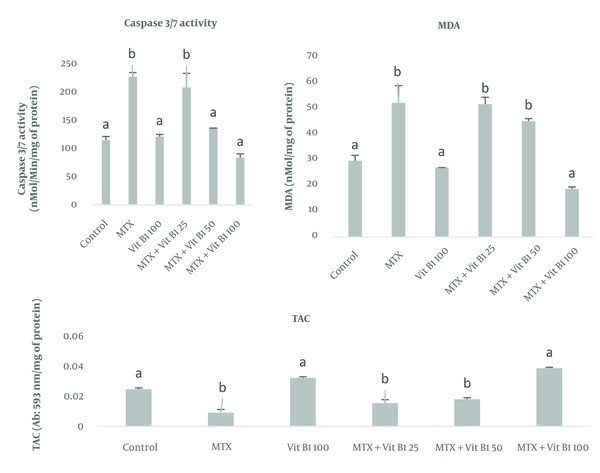
To investigate apoptosis, caspase 3 and 7 enzymes in testicular tissue showed that their levels were highest in the MTX and MTX + VitB1 low-dose groups (P = 0.118). Meanwhile, VitB1 with medium (P = 0.0011) and high (P = 0.0016) doses caused a significant decrease compared to the MTX group, depending on the vitamin dose. To assess oxidative stress, MDA analysis showed that its level was higher in the MTX compared to MTX + VitB1, with a high dose (P = 0.008) causing a decrease in MDA levels to the lowest level. Evaluation of the TAC in testicular tissue showed that its level was lowest in the MTX group. In contrast, the MTX + VitB1 high-dose group showed the highest TAC level compared to the MTX group (P = 0.0013) (Figure 1).
4.1. Histomorphometric Results
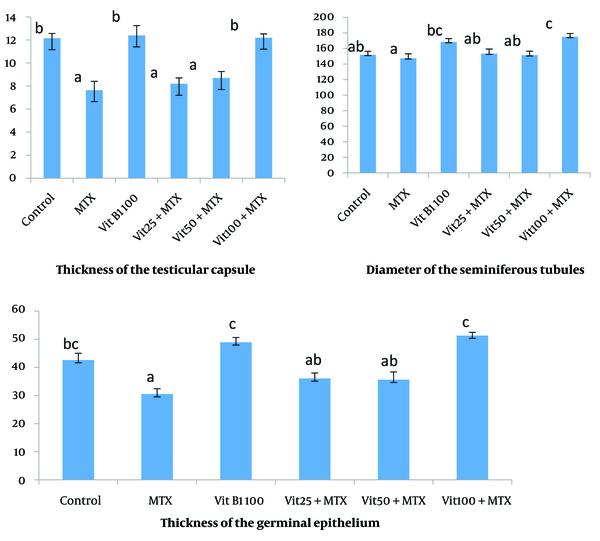
The thickness of the testicular capsule in the MTX group and MTX + VitB1 low and medium doses groups decreased compared to other groups (P = 0.003, 0.000, 0.001). The evaluation of the diameter of the seminiferous tubules and the thickness of the germinal epithelium showed that in the MTX group had a minimum amount, which was significantly different from the groups receiving vitamin B1 100 mg alone and vitB1 100 + MTX (P = 0.048, 0.003) (Figure 2).
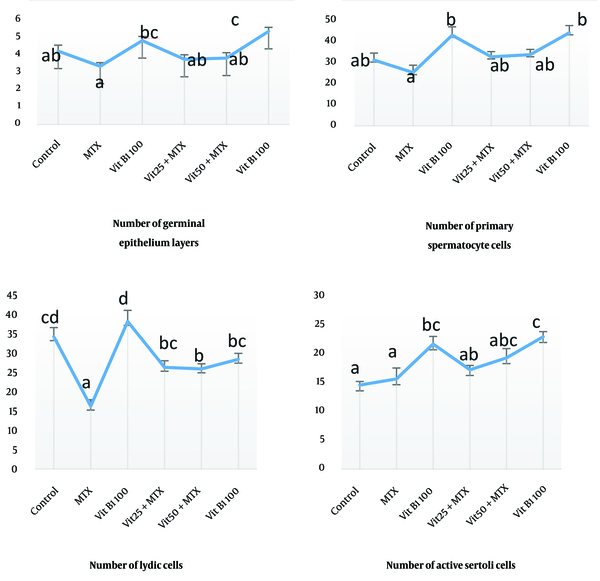
The average number of germinal epithelium cell layers and the primary spermatocyte in the MTX group has a significant decrease compared to the groups control (P = 0.002), MTX + VitB1 alone and high dose (P = 0.000). This study showed that the average number of Leydig cells in MTX was significantly different with all other groups (P = 0.000, 0.008, 0.012, 0.001), and active Sertoli cells (Sertoli cells with accumulation of sperm in their apical surface) in the MTX group had a significant difference compared to VitB1 100 (P = 0.016) and MTX + VitB1 high dose (P = 0.002) (Figure 3).
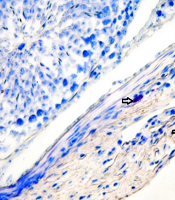
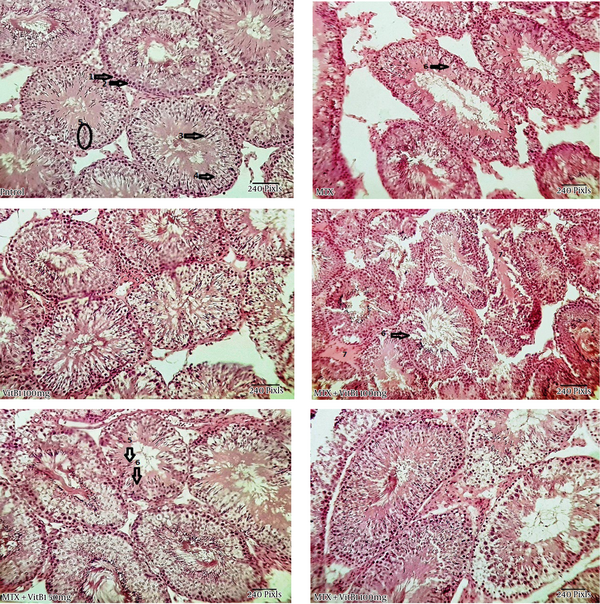
The histological appearance of the testis showed a disintegration of the germinal epithelium, less diameter of seminiferous tubules and large spaces between these tubules in the MTX group. But the MTX + VitB1 groups, according to ascending VitB1 doses, ameliorated (Figure 4).
Study of histomorphometric changes in testicular tissue. Control, healthy group; MTX, methotrexate receiving group, indicated the lower epithelium thickness and germinal epithelium disjunction, VitB1 100 mg/kg; Positive control group, receiving only VitB1, the same as the control group, MTX/VitB1/25 mg/kg, MTX/VitB1/50 mg/kg, MTX/VitB1/100 mg/kg groups, show amelioration VitB1 dose-dependent. Hematoxylin and eosin staining, magnification ×100. (1) Spermatogonia, (2) primary spermatocyte, (3) spermatozoid, (4) spermatid, (5) accumulation of sperm on apical region of Sertoli cell, indicating the active Sertoli cell, (6) germinal epithelium disjunction, and (7) edema.
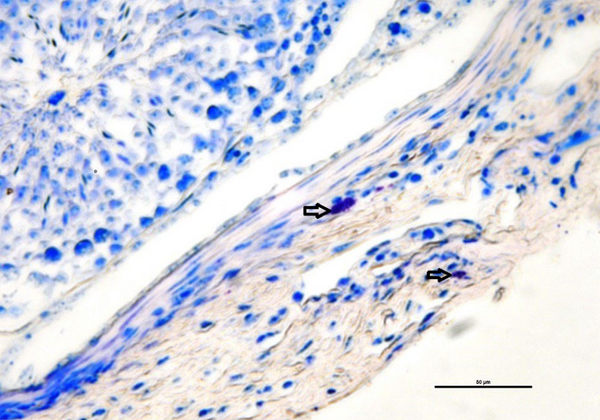
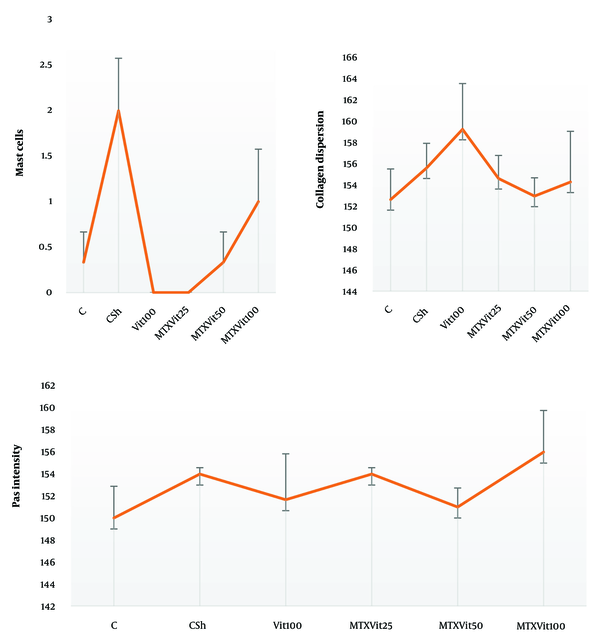
The mean number of mast cells increased in MTX group compared to the other groups (P ≤ 0.05) (Figure 5). Also, there were no significant differences between the groups in the rate of carbohydrate component (PAS reaction) (Figure 6) and in the distribution of collagen fibers in testicular tissue (P = 1) (Figures 7 and 8).
Investigating the average number of mast cells and quantitatively investigating collagen dispersion in testicular tissue shows a high number of mast cells and more dispersion of collagen fibers in the MTX group (csh). However, quantitative investigation of periodic acid-Schiff (PAS) intensity indicates no difference in violet between groups. Dissimilar letters indicate significant differences between groups (P ≤ 0.05).
4.2. Spermatogenesis Examination
The results of spermatogenesis indices showed that the RI in the MTX group decreased compared to the control group (P = 0.98), while in the groups receiving MTX with vitamin B1 at low, medium and high doses compared to the MTX group, it increased respectively (P = 0.989, P = 1.00, P = 0.99).
The Spermiogenesis Index (SI) in the MTX group decreased compared to the control group (P = 0.008), while in the group receiving MTX along with vitamin B1 at doses of 25, 50 and 100, it increased compared to the MTX group (P = 0.109, P = 0.04, P = 0.008).
Regarding TDI, the MTX group showed a decrease compared to the control group (P = 0.949). While in the groups receiving MTX plus vitamin B1, it showed an increase depending on the increase in vitamin dose compared to the MTX group (P = 0.034, P = 0.937, P = 0.448), respectively (Table 1). This study indicates that the Johnsen Score Index significantly decreased (P = 0.001) following MTX treatment compared to the control and VitB1 groups. However, in the MTX + VitB1 (100 mg) group, the Johnsen Score Index significantly increased compared to the MTX group (P = 0.034) (Table 2).
| Groups | RI | SI | TDI |
|---|---|---|---|
| Control | 89.2 ± 3.56 A, B | 94.6 ± 1.69 B | 71 ± 1.9 B, C |
| MTX | 83.38 ± 4.05 A, B | 73.52 ± 6.1 A | 66.5 ± 3.37 A, B, C |
| Vit100 | 94.83 ± 2.26 C, B | 97.26 ± 0.38 B | 68.1 ± 1.1 B, C |
| Vit25 + MTX | 77.42 ± 6.45 A | 89.15 ± 4.38 A, B | 53.37 ± 3.9 A |
| Vit50 + MTX | 82.96 ± 4.17 A, B | 92.86 ± 1.33 A, B | 61.2 ± 1.73 A, B |
| Vit100 + MTX | 88.5 ± 2.17 A, B | 97.6 ± 0.33 B | 75.16 ± 1.28 C |
Investigating of Spermatogenesis Examination Indexes a
5. Discussion
Testicular toxicity is an important side effect for MTX (24). Chemotherapeutic agents have cytotoxic effects on mitotically active cells, such as testicular germ cells, leading to gonad toxicity by disrupting the regulated balance between spermatogenesis and apoptosis processes (25). The results of our evaluation about apoptosis showed an increase in the MTX group but, by increasing the dose of vitaminB1 in the treatment groups the amount of apoptosis decreased. This result accommodated with the previous research, that thiamine reduces apoptotic effects on cells by affecting active P53 (26). In the present study, the MDA decreased by vitamin B1 dose-dependently. In reverse, vitamin B1, increased the TAC against the MTX detrimental effects. Previous research has shown that MTX increases inflammation, alters energy sources in the germ cell lineage (27), and inhibits DNA synthesis by affecting the cell cycle (28). On the other hand, it has been found that thiamine has a therapeutic effect on cellular stress by reducing the cellular levels of free radicals and preventing protein oxidases (29, 30). The testicular capsule, which contains blood vessels and nerves, is a tough outer layer that surrounds the testicles and helps protect this tissue (31). Chemotherapy can cause inflammation and damage to the testicular capsule, leading to pain, swelling, and other problems (32). Our study was shown that the histomorphometrical parameters decrease in MTX group compared to thiamine receiving groups, this finding was in line with the other researches (33, 34). High levels of free radicals in testicles negatively affect the germ cells, spermatozoa, somatic cells and testicular structure by pathologically affecting the cellular DNA, RNA and protein structures (35). In the testicular tissue, Sertoli cells have a protective and supportive role for spermatogenic cells and are very resistant to oxidative factors. By secreting testosterone, Leydig cells control the physiological interactions of Sertoli cells (36). Therefore, it is more reasonable to conclude that MTX reduces the endocrine status and negatively affects the participation of Sertoli cells in the processes of intact spermatogenesis and spermatogenesis, this can ultimately promote cell degeneration (27). Mast cells act as pro-inflammatory cells in the testicular tissue, and in the present study shown that the number of these cells in the MTX group increased significantly compared to the other groups. The degranulation of mast cells leads to the secretion of histamine and chemotactic factors in response to acute physical and chemical stress, which leads to an increase in the permeability of blood vessels and recall of immune cells, and as a result, it can lead to infertility in the testicular tissue (37). A study showed that the mast cells interact with fibroblasts in a manner that leads to fibroblast activation and subsequent extracellular fibrosis. Furthermore, the notion that myofibroblasts represent a critical fibroblast phenotype in sclerosing disorders has also gained considerable support (38). Spermatogenesis is a highly active process capable of producing more specialized cells called sperm (39). Growth capacity and germinal epithelium differentiation are determined by spermatogenesis indices. In this study, it was shown that the oxidative stress caused by MTX decreased the spermatogenesis indices, and receiving VitB1 along with MTX compensated. Indeed, with the massive cell division, proliferation and differentiation during spermatogenesis, significant free radicals are generated. Therefore, by balancing free radical production and tissue antioxidant capacity, it maintains testicular homeostasis, leading to normal spermatocytogenesis, spermatogenesis, and spermiogenesis (40). Increasing the body’s antioxidant capacity and eliminating free radicals is the mechanism of this protective process, which acts synergistically and creates an effective barrier against destructive oxidation (39). The use of a higher dose of MTX, the complete examination of inflammatory parameters of testicular tissue, and the examination of the protective effect of thiamine in the short term, such as one week, and the long term, such as two months, are among the limitations of this study. It is also suggested that in the continuation of this study, the ultramicroscopic structure of Sertoli cells and spermatogonia should be examined.
5.1. Conclusions
The present study can claim that thiamine is partially responsible for the prevention of testicular injury during chemotherapy and thus the preservation of fertility. It also revealed that the protective effect of thiamine was dose-dependent, and the maximum dose used in this manuscript had no toxic effect.