1. Background
Acinetobacters are Gram-negative bacillus or coccobacillus bacteria that do not have fermentation potential and prefer to live in a humid environment (1). These bacteria are characterized by fewer nutrient requirements, survival in adverse conditions, dry surfaces, and also the aquatic environment. Acinetobacter baumannii, the most important species of this genus, is rarely the cause of severe infections in people with healthy immune levels and is less commonly known as the healthy person’s normal flora (2, 3).
The rate of colonization of this type of bacteria is increasing in people receiving extensive or antimicrobial treatment and in long-term hospitalized patients (2, 4). The respiratory system is the most common site for Acinetobacter infection, and high rates of colonization are seen in people who have undergone tracheostomy (5).
This species is involved in the development of critical diseases in hospitalized patients in various parts of the hospital such as peritonitis, endocarditis, meningitis, respiratory tract infections, burns and toxic septicemia (1).
Acinetobacter baumannii with multiple drug resistance refers to species that resist at least three major antibiotic groups, including beta-lactam, aminoglycoside, and quinolone (6).
The resistance of Acinetobacter baumannii to antimicrobial agents may be intrinsic or through genetic factors including enzymatic alteration, mutation of target genes, alteration of the permeability of the outer membrane and increased expression of efflux pumps (7, 8). This infection is widespread in health centers because most cases of nosocomial infections are transmitted by staff (9). Acinetobacter strains have become resistant to the majority of antibiotics in recent years. Most of the Acinetobacter baumannii strains are resistant to Ampicillin, amoxicillin-clavulanic acid, anti-staphylococcal penicillin, Wide spectrum cephalosporin (except ceftazidim and cefepime), tetracycline, macrolides, Rifampicin, and chloramphenicol. Resistance to non-carbapenems beta-lactamases in this bacterium is often associated with the over-production of cephalosporin. The treatment of Acinetobacter infections is considered a significant health problem in many countries and this problem may have adverse effects on clinical outcomes and therapeutic costs (10, 11).
Due to limited studies and reports on the drug resistance pattern of Acinetobacter isolates in western Iran, attention is needed to the role of this bacterium as a potentially dangerous agent in nosocomial infections. Comprehensive information on the prevalence, type, and quantity of Acinetobacter bacteria in each treatment unit such as hospitals is essential to reduce contamination with Acinetobacter baumannii infection in desired sources such as equipment and staff hands.
2. Objectives
To prevent the spread of antibiotic resistance, it is necessary to identify patterns of antibiotic susceptibility in each geographical area. Given the lack of knowledge about the prevalence and antibiotic resistance of Acinetobacter infections in Kermanshah, Iran; this study aimed to determine the incidence of Acinetobacter strains isolated from ICU equipment and patients.
3. Methods
3.1. Sampling Procedures
In this descriptive-analytical study, random sampling was performed from the Respiratory ICU, Thoracic ICU and General ICU of Imam Reza Hospital (750 beds, 29 sections, governmental, general high direct referral rate admission) during summer and autumn 2017.
Samples were obtained from three groups:
1. Hospitalized patients (sputum specimen, endotracheal tube, wound, and patients)
2. ICU staff (hand of nurses, nurse assistants, and medical students)
3. Hospitalized equipment (tracheal tube, catheter, etc.) and equipment available in ICU (Respiratory-General and Thoracic)
Sampling was performed using sterile swab impregnated with tryptone soy broth from dry surfaces of the beds, staff hands, external wall of equipment, bed handles, desks, and all other items to check for contamination.
Swabs were inserted into a TSB culture medium and immediately transferred to the microbiology laboratory, and samples were immediately cultured in blood agar medium.
The exclusion criteria for entry into the study was that the bacterial culture was not positive.
3.2. Laboratory Methods
After the inoculation of clinical samples on MacConkey agar and blood agar medium, cultured plates were incubated at 37ºC for 24 - 48 hours. Following gram staining and observation of gram-negative cocci and diplococcus, an oxidase test was performed.
Definitive diagnosis was made by biochemical tests, culture on Macconkey agar and incubation at 37ºC and 42ºC, citrate test, motility test, and culture on OF medium containing glucose.
3.3. Antibiotics
Standard disk diffusion assay (Kirby-Bauer) was used for 10 antibiotics: cefepime, cefazolin, trimethoprim, gentamycin, pipracilin, imipenem, ciprofloxacin, ampicillin, ceftriaxone, and ceftazidime. McFarland’s Tube 0.5 was used to make suspension of bacterial colonies and, after impregnating the sterile swabs with the suspension, was spread on the Muller-Hinton agar medium. Next, antibiotic discs were placed at a standard distance from each other.
The samples were then incubated at 37ºC for 24 hours, and finally, the zone of inhibition of bacterial growth (diameter of inhibition) was measured. The results were recorded as sensitive, intermediate, and resistant for each antibiotic according to the relevant instructions.
3.4. Statistical Analysis
SPSS 21 (IBM) software and chi-square test were used. P values of 0.05 were considered as significant.
4. Results
In this descriptive-analytical study, a total of 200 samples were collected from staff and different wards of the hospital, out of which 76 (36%) were from patients and sputum, 51 cases (25.5%) from staff. and 73 (36.5%) from equipment.
4.1. Patient Sampling Results
A total of 76 samples were taken from patients admitted to ICU wards of Imam Reza Hospital. 50.7% of patients (38 patients) were male, and 49.3% (37 patients) were female. 46.7% of the specimens (35 cases) were from the trachea, 40% (30 cases) from the hand, 12% (9 cases) from the sputum and 1.3% (1 case) from the injuries of patients.
17.4% (12 cases) of ICU patients had an immune deficiency, 2.9% (2 cases) diabetes, 65.2% (45 cases) other diseases (such as heart disease, cirrhosis, trauma) and 1.45% (10 cases) had lung disease. No significant relationship (P < 0.05) was found between isolation of Acinetobacter from sputum and trachea in patients with history of antibiotic use (P = 0.25) and underlying disease (P = 0.14) and intubation (P = 0.226) (Table 1). Acinetobacter baumannii showed 100% resistance to cefepime, cefazolin, trimethoprim, gentamycin, pipracilin, imipenem, ciprofloxacin, ampicillin, ceftriaxone, and ceftazidime.
| Acinetobacter | P Value | |||
|---|---|---|---|---|
| Isolated | Not Isolated | Total | ||
| History of antibiotic use | 0.255 | |||
| Yes | 30 (44.8) | 37 (55.2) | 67 (100) | |
| No | 0 (0) | 3 (100) | 3 (100) | |
| Underlying disease | 0.140 | |||
| Immune deficiency | 8 (66.7) | 4 (33.3) | 12 (100) | |
| Lung disease | 5 (50) | 5 (50) | 10 (100) | |
| Diabetes | 0 (0) | 2 (100) | 2 (100) | |
| Other | 16 (35.6) | 29 (64.4) | 45 (100) | |
| Tracheal intubation | 0.226 | |||
| Yes | 29 (46) | 34 (54) | 63 (100) | |
| No | 1 (14.3) | 6 (85.7) | 7 (100) | |
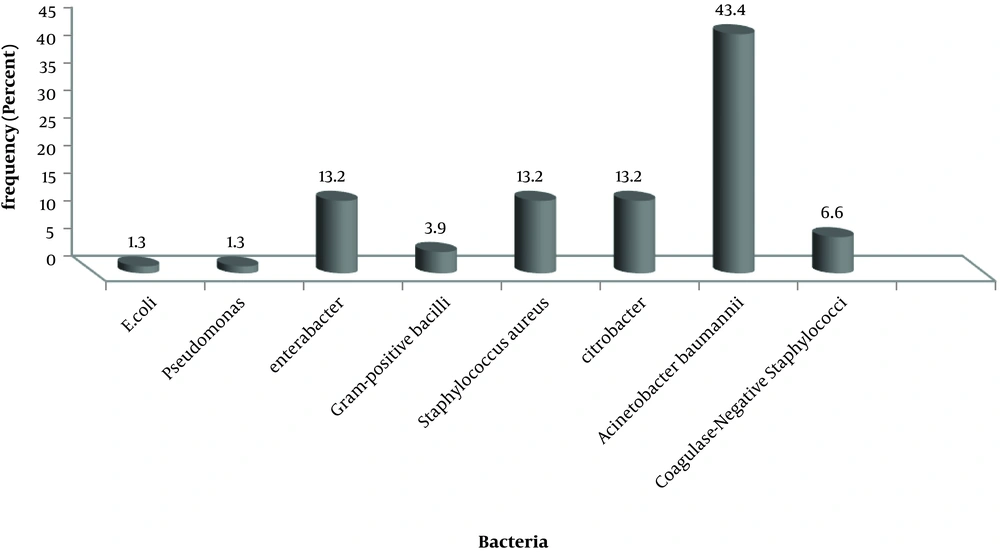
Acinetobacter baumannii (43.3%), Citrobacter, Enterobacter and Staphylococcus aureus, Coagulase-negative Staphylococcus, Gram-positive bacilli, and E. coli were isolated from patients (Figure 1).
4.2. Staff Sampling Results
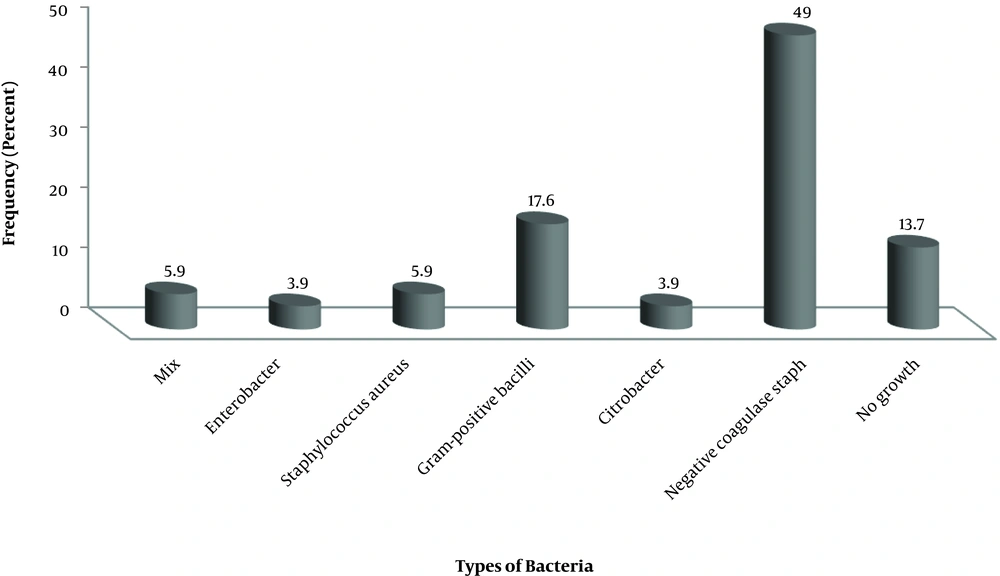
A total of 51 samples were taken from the hand of hospital staff, out of which 60.8% were female, and 39.1% were male. The most isolated microorganisms were 49% with Staphylococcus aureus (Figure 2). In this sampling, no case of Acinetobacter was isolated from the personnel.
4.3. Equipment Sampling Results
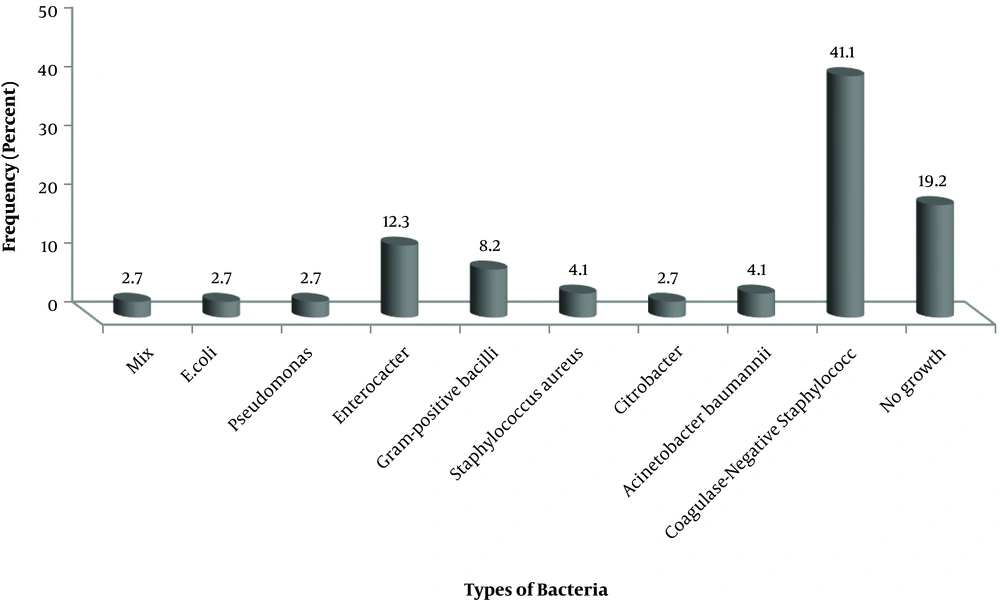
The sampling of beds, hospital trolleys, scissors, pens, suction, and metal files in ICU wards was performed. Out of 73 specimens, Acinetobacter baumanii (4.1%), Coagulase-negative staphylococci (41.1%) and Enterobacter (12.3%) and gram-positive bacilli (8.2%) were isolated from the patient’s case file in ICU I and the suction adjustment screw in ICU II and surgical ICU desk (Figure 3). Acinetobacter baumannii isolated from equipment showed 100% resistance to antibiotics cefepime, cefazolin, trimethoprim, gentamycin, pipracilin, imipenem, ciprofloxacin, ampicillin, ceftriaxone, and ceftazidime.
5. Discussion
The findings of the antimicrobial resistance analysis showed that all isolates are resistant to cefepime, cefazolin, trimethoprim, gentamycin, pipracilin, imipenem, ciprofloxacin, ampicillin, ceftriaxone, and ceftazidime.
Acinetobacter baumannii’s multiple resistance has created several medical problems in the treatment of patients infected with this organism and many variations in resistance to different antibiotics were induced by environmental factors and specific antibacterial application patterns. The development and spread of these resistant organisms in various parts of the hospital seems to be affected by variations in antibiotic usage patterns and lack of adequate resources to manage hospital infections (1, 12-17). Hospitalized pathogenic bacteria may have different patterns of antibiotic resistance from one country to another, or in different regions of the country.
Previous reports showed that between 30% and 83.9% of Acinetobacter baumannii strains are multidrug-resistant (18). Studies in Asia and the Middle East show the prevalence of Acinetobacter baumannii with multiple drug resistance in these regions (19-21). These studies have also demonstrated increased resistance to cephalosporins (cefepime, ceftazidime), extended-spectrum penicillins and carbapenems (imipenem, meropenem), aminoglycosides (gentamicin, tobramycin) and fluoroquinolones (ciprofloxacin) (22).
Ferreira et al. (23) in Brazil reported that, In the disk diffusion agar method, 68% of isolates are multidrug-resistant and 79% are resistant to carbapenem, and the resistance reported in this study is lower than the present study. In a survey by Hujer et al. (24) on Acinetobacter strains isolated from military and civilian patients of the Iraq-Afghanistan war, 89% of isolates were resistant to at least three classes of antibiotics using the agar disk diffusion method, which is a criterion for determining multidrug resistance. In this study, more than 90% of samples were resistant to ciprofloxacin and extended-spectrum cephalosporin, which is consistent with the results of our study. Karlowsky et al. (25) reported that 90% of the strains are sensitive to meropenem. In another study, it was reported that of the 32 Acinetobacter baumannii strains examined, all isolates were susceptible to imipenem, which is in sharp contrast to the results of our study (26). Acinetobacter species appear to be resistant to Imipenem over time.
In a 2009 study in Iran, 60 Acinetobacter strains were isolated from 400 hospitalized patients. These strains showed the highest resistance to amikacin, tobramycin, ceftazidim ciprofloxacin, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, and imipenem, respectively, in disk diffusion agar method (27). In another study in 2003 using the disc diffusion agar method, of the 52 strains, all isolates were resistant to pipracilin, piperacillin-tazobactam, ticarcillin-clavulanic acid, cefepime, cefotaxime, ceftazidime, ceftriaxone, gentamycin, and aztreonam. the resistance to tobramycin, ciprofloxacin, ampicillin-sulbactam, co-trimoxazole, and amikacin was observed in 5%, 8%, 55%, 66% and 74% of the strains, respectively (28). In another study in 2007, 15 Acinetobacter strains were isolated from 400 ICU patients. Antibiogram results of these samples by disk diffusion agar showed that 26.6% of the strains were resistant to imipenem (29). Fazeli et al. (30) reported that all 21 Acinetobacter isolates had multidrug resistance, which is consistent with the results of our study.
In the present study, 100% of isolates were multidrug-resistant, which is much higher than previous studies in other parts of Iran. Also, Vahdani et al. (31) reported that Acinetobacter baumannii resistance to Meropenem is 99% and to ciprofloxacin and levofloxacin 98, which is consistent with our findings. Esmaeilzadeh Ashini reported that in the disc diffusion agar method 100% of the strains were resistant to streptomycin and nalidixic acid and more than 70% of the isolates were resistant to tetracycline, ampicillin, and gentamicin antibiotics (32). In another study similar to this method, all strains were resistant to gentamycin, ciprofloxacin, pipracilin, cefotaxime, ceftazidime, and tetracycline (33).
5.1. Conclusion
Overall, the results of this study indicate high numbers of Acinetobacter baumannii isolates with multiple drug resistance, but these findings need to be confirmed with a more significant amount of isolates. Therefore, infection control actions are necessary to eliminate potential sources of infection with Acinetobacter baumannii and to prevent transmission of infections to patients through the hands of hospital staff and equipment.