1. Background
Liver and colon cancers are prevalent cancers and respectively the second and third leading causes of mortality across the world. Approximately 700,000 deaths due to colon cancer were reported in the United States in 2019, while liver cancer led to the death of 30,000 individuals in 2018 (1-6).
Reports have indicated that numerous plants have anticancer effects. Galium verum belongs to Rubiaceae family and is also known as ‘Lady’s Bedstraw’ or ‘Yellow Bedstraw’ (7, 8). Galium verum contains anthraquinone compounds, tannins, phenolic compounds, and saponins. In Iran, the genus Galium is represented by almost 50 species, one of which is Galium verum (known as Shir Panir in Persian) (9). Galium verum is used for various purposes, including kidney and bladder swelling treatment (1), wound healing, psoriasis and rheumatism treatment, and as a sedative. According to the literature, this plant may help the improvement of various cancers, including gastrointestinal, liver, cardiovascular, kidney, and stomach cancer (10-13). In addition, other findings have shown that the methanolic extract of Galium verum has cytotoxic effects on several cancers, such as breast, larynx (14), head and neck, liver (14), and blood cancer (14). Several studies have also indicated the toxicity of the methanolic, chloroform, petroleum ether, and aqueous extracts of 25 plants of the Rubiaceae family on colon cancer (HT29) and fibroblast (HSF) cell lines. However, no antiproliferative effects have been observed in some of these plants (5). In another study, the cytotoxic effects of the ethanolic, methanolic, and aqueous extracts of some medicinal plants of the Rubiaceae family were confirmed on colon (HCT116) and liver (HepG2) cancer cell lines (15). In contrast, previous studies have revealed that the aqueous and ethanolic extracts of some members of the Rubiaceae family have no effects on colon adenocarcinoma (LS/174 T) (16).
Several studies have confirmed the non-toxicity of the ethanolic extract of Argemone mexicana terminalia bellerica on the liver cell line, as well as the effects of the methanolic, chloroform, and aqueous extracts of Dicranopteris linearis on the colon cancer cell line (HT29) (17). Moreover, it has been reported that Galium verum could heal wounds and inflammations in non-cancerous cells. Several studies have also indicated that some medicinal plants (e.g., Rubiaceae family) have significant effects on the proliferation of fibroblasts and wound healing (11).
2. Objectives
3. Methods
3.1. Extraction
In this study, Galium verum with the herbarium code of 4521 TMRC for the entire plant was collected and identified by Traditional Medicine and Materia Medica Research Center of Shahid Beheshti University of Medical Sciences in Tehran, Iran. After drying and grinding all the plant parts, fractionation was performed using chloroform and/or petroleum ether solvents (Merck, Germany) and the maceration method. Afterwards, two portions of the obtained powder (30 g) were separated and mixed with 300 milliliters of petroleum ether and 300 milliliters of chloroform and placed on a shaker (Faraz Teb Azma Company, COMBI) for 24 hours. Following that, the obtained solutions were filtered and concentrated using a rotary evaporator (Heidolph Company), and the concentrated samples were dried at room temperature.
3.2. Cell Culture and Cell Treatment
Human hepatocellular carcinoma (HepG2) and human colorectal adenocarcinoma cell lines (HT29) were purchased from the Iranian Genetic Resource Center and stored frozen in a nitrogen tank. The HepG2 and HT29 cell lines were divided into the control and treatment groups (concentrations of 100, 50, 25, 12.5, 6.25, and 3.125 µg/mL).
3.3. MTT Assay
In order to perform the MTT assay, 100,000 cells were obtained from the HepG2 and HT29 cancer cells separately and initially cultured in 96-well plates on the DMEM culture medium containing 10% FBS for 24 hours. The treatments were performed with concentrations of 100, 50, 25, 12.5, 6.25, and 3.125 µg/mL. After 72 hours of exposing the cells to the fractional extract, the supernatant was removed, and 80 microliters of the fresh culture medium and 20 microliters of MTT were added to each well. At the next stage, the plates were incubated for four hours, the culture medium was completely evacuated, and 200 microliters of the DMSO solution was added to each well. After the complete dissolution of the purple formazan crystals by the DMSO solvent, the absorbance rate of the samples was measured at 570 nanometers using the ELISA reader (Viragen Co., Epoch).
3.4. Statistical Analysis
Data analysis was performed using the analysis of variance and Tukey’s range test in the GraphPad Prism software version 8 at the significance level of P ≤ 0.05.
4. Results
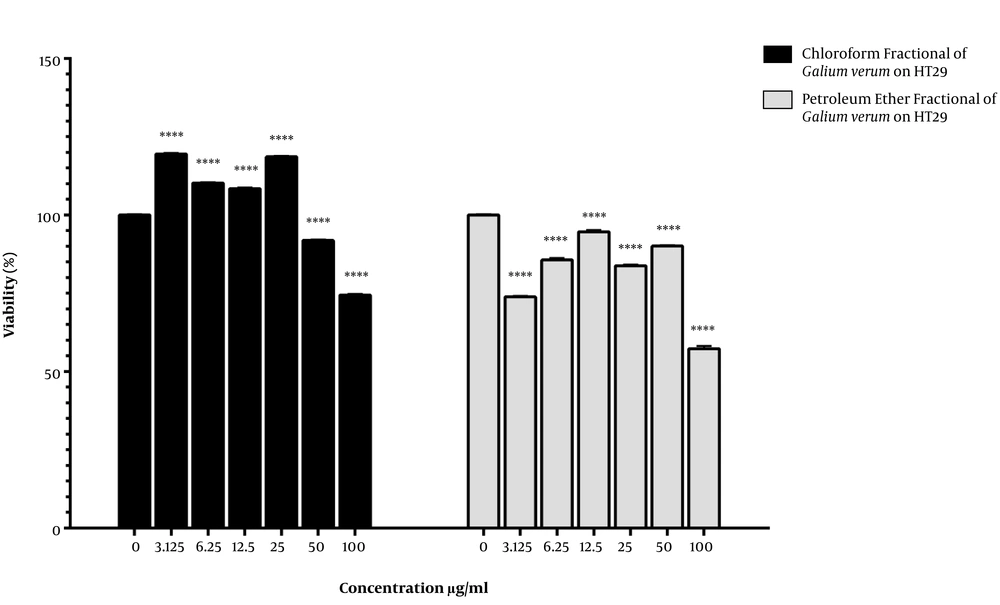
As is shown in Figure 1, cell viability increased in the HT29 cancer cells treated with the plant chloroform fractional extract at the concentrations of 25, 12.5, 6.25, and 3.125 µg/mL. In contrast, treatment with the concentrations of 100 and 50 µg/mL significantly decreased cell viability compared to the control group (IC50 > 100 µg/mL). In the treatment with the petroleum ether fractional extract (100, 50, 12, 25.5, 6.25, and 3.125 µg/mL), cell viability significantly decreased compared to the control group (IC50 > 100 µg/mL).
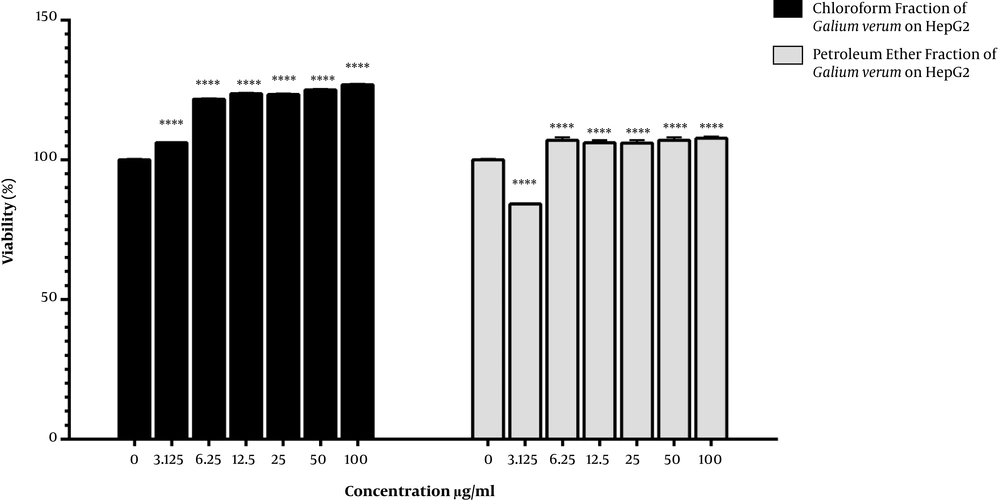
Figure 2 depicts the viability of the HepG2 cancer cells treated with the chloroform and petroleum ether fractional extracts. Accordingly, the cell viability increased in the HepG2 cancer cells treated with the chloroform fractional extract at all the selected concentrations compared to the control group. On the other hand, treatment with the petroleum ether fractional extract significantly decreased the cell viability only at the concentration of 3.125 µg/mL.
5. Discussion
According to the results of the present study, the fractional petroleum ether extract of Galium verum had cytotoxic effects on HT29 at all the administered doses, while it affected the HepG2 cancer cells only at the concentration of 3.125 µg/mL. Although the chloroform fractional extract had cytotoxic effects on HT29 at the concentrations of 100 and 50 µg/mL, it increased the cell viability of the HepG2 cancer cells.
In line with our findings, Manosroi et al. investigated the effects of the methanolic, chloroform, petroleum ether, and aqueous extracts of 25 plants of the Rubiaceae family on colon cancer cell lines (HT29) and fibroblasts (HSF) using the MTT assay. According to the obtained results, some of the plants had anticancer effects, while no antiproliferative effects were observed in others. Furthermore, the petroleum ether and chloroform extracts were reported to be the best solvents for extraction (5). In another study, Itharat et al. assessed the effects of the ethanolic and aqueous extracts of 11 medicinal plants belonging to the Rubiaceae family with the concentrations of 100, 50, 25, 10, 1.5, 0.5, and 0.1 µg/mL on colon adenocarcinoma (LS/174 T) using the MTT assay. In the case of most of the plants, the aqueous extract with the concentration of 15.6 µg/mL as the IC50 was more toxic compared to the ethanolic extract. However, the aqueous extracts of Dioscorea birmanica and Siphonodon celastrineus had no effects on the colon cancer cell line (16).
In another research, Talib Hussain evaluated the effects of the ethanolic extract of Terminalia bellerica on the liver cancer cell line (Hep-3B), as well as the effects of the methanolic, chloroform, and aqueous extracts of Dicranopteris linearis on the colon cancer cell line (HT29) using the MTT assay. The obtained results suggested that the aqueous extracts had no effects on the HT29 and HepG2 cancer cell lines (17).
In the present study, the petroleum ether fractional extract had cytotoxic effects on the HT29 cancer cells at all the determined concentrations, while it affected the HepG2 cancer cells only at the concentration of 3.125 µg/mL. Consistent with this finding, other studies have also indicated that Galium verum extracts have cytotoxic effects on several cancers, including breast, larynx (8), head and neck, liver (14), and blood cancer cell lines (18).
Schmidt et al. (14) investigated the cytotoxic effects of the aqueous extract of Galium verum at the concentrations of 50 and 100 µg/mL on larynx, liver, and head and neck cancer cells (HLaC78, Hep2, and FADU) using the MTT assay, western blot, and real-time polymerase chain reaction (PCR). According to their findings, the plant could be used as a preventive measure for cancer treatment (8, 10-12, 14). In another study, Zhao et al. examined the cytotoxic effects of the effective substances of Diosmetin from Galium verum at the oral doses of 400, 200, and 100 mg/kg on mice with thymic cancer using western blot, MTT assay, and flow cytometry. No mortality was observed in the mice treated with Diosmetin, and Diosmetin was reported to exert protective effects on the thymus against tumor invasion, while reducing the proliferation of the thymic cells, increasing the thymus weight, and inhibiting tumor growth in the treated mice compared to the controls (19).
In another study, Siew et al. assessed the cytotoxic effects of the ethanolic, methanolic, and aqueous extracts of some medicinal plants of the Rubiaceae family on the colon (HCT116) and liver (HepG2) cancer cell lines using the MTT assay. The obtained results indicated that the methanolic extract of the leaves with the IC50 concentration of 31.5 µg/mL could significantly decrease cell viability (15). In another study, the effect of the methanolic extract of some medicinal herbs of the Rubiaceae family on HepG2 was investigated using the MTT assay, and the viability of the cancer cells treated with Smallanthus sonchifolius leaf at the concentration of 58.2 µg/mL as the IC50 was reported to decrease (1).
Chiranjeevi et al. investigated the effects of the ethanolic, ethyl acetate, and petroleum ether extracts of Lindernia madayiparense of the Linderniaceae family at the concentrations of 1,000, 500, 250, 125, and 62.5 µg/mL on the HepG2 cell line using the MTT assay, reporting that the petroleum ether extract with the concentration of 149 µg/mL as the IC50 exerted the most toxic effect (20). Similarly, Deivayanai et al. evaluated the effects of the ethanolic and chloroform extracts of various plants with the concentrations of 10, 20, 30, 40, and 50 µg/mL on the HepG2 cell line using the MTT assay, reporting the reduction of the viability of the cancer cells treated with both extracts (21).
The results of the present study indicated that the chloroform fractional extract of the entire Galium verum plant had no cytotoxic effects on the HepG2 and HT29 cancer cell lines, except at the concentrations of 50 and 100 µg/mL. However, the petroleum ether fractional extract exerted cytotoxic effects on the HT29 cancer cells at all the determined concentrations, while also affecting the HepG2 cancer cells at the concentration of 3.125 µg/mL.
In another research, Buk-Gu Heo examined the effects of the methanolic extract of Uncaria rhynchophylla of the Rubiaceae family at the concentration of 500 µg/mL on the HT29 cell line using the MTT assay, reporting that the presence of polyphenols and high biological activity of the extract led to cell growth inhibition in a dependent manner (22). Furthermore, Jaya Kumar investigated the inhibitory effects of the hexane extract of Morinda pubescens of the Rubiaceae family at the concentrations of 250, 100, 50, and 25 μg/mL on the HepG2 cell lines using the MTT assay. The results of the mentioned study demonstrated that the hexane extract of the leaves significantly decreased cell viability at all the concentrations (23). The other studies in this regard have also confirmed the effects of the Galium verum extract on wound healing and psoriasis (11).
According to the literature, compounds as anthraquinones, flavonoids, triterpenoid saponins, glycoside monoterpenes, erythroids, and phenols contain bioactive components with cytotoxic effects of variable grades (7, 23). The findings of the current research suggested that the petroleum ether extract had more significant cytotoxic effects than the chloroform extract due to the presence of non-polar compounds, such as triterpenoid saponins (24-27).
Further investigation is required in order to evaluate the effects of the fractional extracts of petroleum ether and chloroform of Galium verum on the liver cancer cells, especially in the fields of phytochemicals and instrumental analysis. The present study examined the effects of the petroleum ether and chloroform fractional extracts of Galium verum on the survival of liver and colon cancer cells in the cell culture medium, and the results indicated that the fractional petroleum ether extract had cytotoxic effects on the HT29 cancer cells at all the administered doses, while it affected the HepG2 cancer cells only at the concentration of 3.125 µg/mL.