1. Introduction
Rituximab is an anti-CD20 monoclonal antibody, which is used for the treatment of various autoimmune diseases (1). Today, rituximab is also applied in the treatment of multiple sclerosis (MS). However, the use of this drug in highly active MS is considered to be challenging and controversial. The present study aimed to describe the case of a patient with a fulminant radiological presentation, which was effectively controlled using ZytuxTM (rituximab, AryoGen Pharmed) (2).
2. Case Presentation
A 21-year-old woman initially presented with vertigo in July 2015, which resolved spontaneously. After four months, the patient experienced vertigo and blurred vision in both eyes, which also resolved spontaneously without a physician visit. However, the patient had complaints of loss of balance after three months, for which she referred to a physician. Magnetic resonance imaging (MRI) was requested, which revealed the development of numerous periventricular plaques. The patient underwent pulse therapy and improved markedly. However, she refused to continue the treatment.
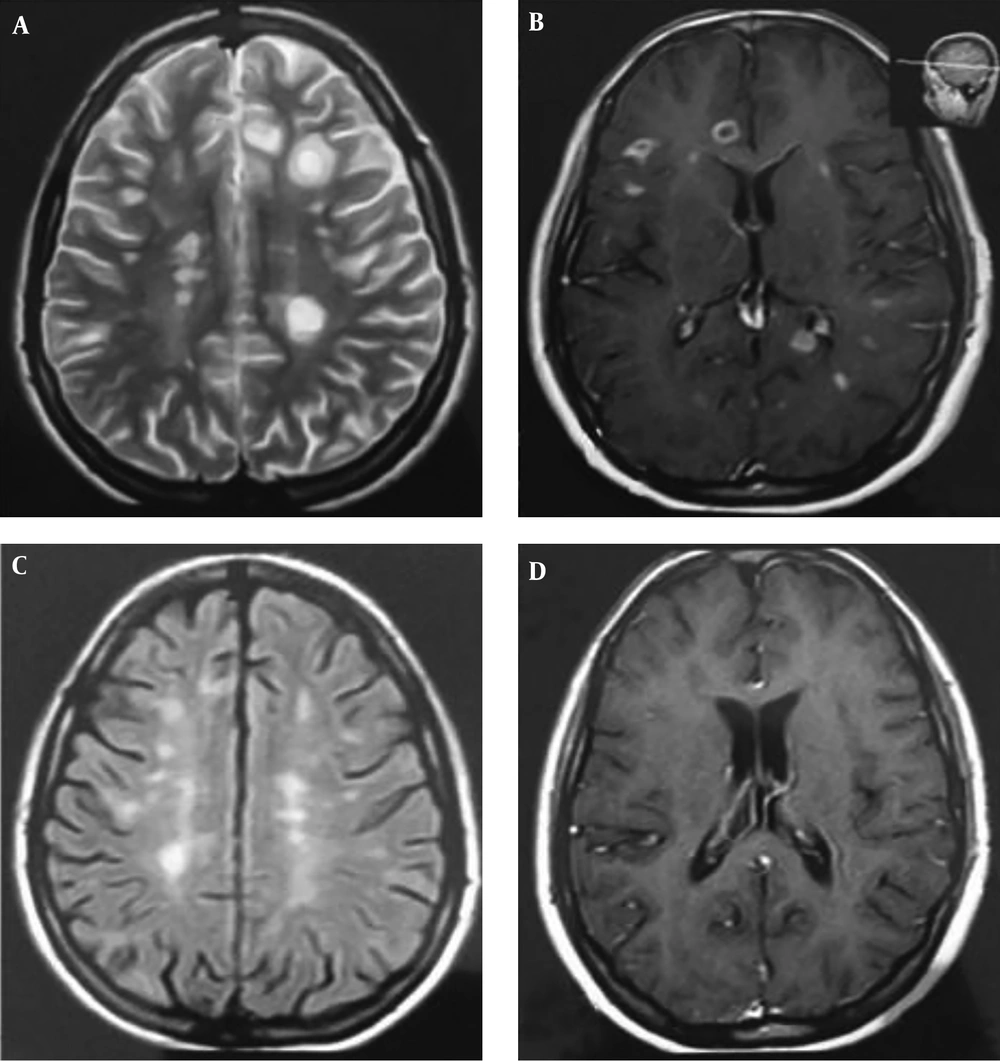
Routine laboratory tests (e.g., blood biochemistry) were normal, while the vasculitis tests and anti-aquaporin 4 antibody were negative. The patient had no history of medication use, and her medical history and family history were also unremarkable. The patient was visited in August 2015 with no neurological complaints, and her neurological examination was also intact. The MRI indicated extensive periventricular involvement with multiple enhancing lesions (Figure 1A and 1B). Therefore, she was hospitalized and received pulse methylprednisolone (1 g) daily for five days. Afterwards, the patient was administered with ZytuxTM (1 g) for two weeks. In addition, controlled MRI was performed approximately six months after the ZytuxTM administration, which showed a significant reduction in the plaque volume, while none of the plaques enhanced (Figure 1C and 1D). Considering the marked improvement, the patient was advised to receive ZytuxTM (1 g) every six months. The patient was relapse free and other routine follow ups by MRI were not revealed any new plaque.
3. Discussion
Rituximab is an anti-CD20 monoclonal antibody, which is commonly used for the treatment of various autoimmune diseases (1). Since the role of B cells in the pathogenesis of MS has attracted the attention of researchers, numerous reports have been focused on the use of the drug in the treatment of MS, while it is also administered in relapsing-remitting, primary progressive, and secondary progressive MS (1). Moreover, some studies have mentioned the application of rituximab in the case of other concomitant autoimmune diseases than MS (3) or when the patients administered with natalizumab are unable to continue the treatment for any reasons (e.g., high John-Cunningham virus index) (4). Due to the marked effect of the drug on B cells, rituximab has also been used in the treatment of fulminant MS (5).
The treatment of highly active MS is rather challenging (6). Chemotherapy and stem cell therapy have been proposed for the treatment of the cases diagnosed with highly active MS. Despite the controversy over the use of rituximab for the treatment of aggressive or highly active MS (6), some reports have been published in this regard (5).
3.1. Conclusions
The patient described in the present study is an example of the successful treatment of MS using rituximab.