1. Background
Citrus grandis originates in India and Malaysia (1). Historically, plants with the potential of antibacterial properties have been used in the treatment of infectious diseases. Today, the increased resistance of pathogenic bacteria against antibiotics has led researchers to screen medicinal plants with antimicrobial properties (2, 3). The standard methods used for the extraction of raw materials from various parts of medicinal plants are aimed at achieving secondary metabolites with antimicrobial properties and pharmaceutical processing in the field of medicine (4).
C. grandis is a thin tree, with the height of 4.5 - 9 meters and angular branches, thin thorns, and single flowers with broad petals, spherical fruits, and a large number of seeds in the fruits. In the past, C. grandis was used to improve appetite and as an anti-venom agent. The extract of this plant has antioxidant and antimicrobial properties (5). Flavonoids with antimicrobial properties (e.g., naringin and hesperidin) are found in the fruit skin of citrus. The antimicrobial activity of tannins is attributed to the inactivation of enzymes and cellular membrane proteins (6, 7). Bacterial resistance against compounds such as plant extracts has been reported to be slower compared to the drugs produced from these compounds (8, 9).
Antioxidants are polyphenol compounds, which are found in all parts of plants, such as the skin, leaves, stems, fruits, roots, and seeds (10). Antioxidants reduce the risk of cardiovascular disease and prevent the progression of cancers (11). In general, Gram-negative bacteria are resistant to herbal antimicrobial compounds unless used at high concentrations (8).
2. Objectives
The present study aimed to investigate the antibacterial and antioxidant activities of the aqueous and alcoholic extracts of Citrus grandis skin against 10 human pathogenic bacteria and evaluate the presence and absence of secondary metabolites, including alkaloids, tannins, and saponin, in-vitro.
3. Methods
3.1. Chemical Materials
The DPPH assay, Mueller-Hinton agar (MHA), nutrient broth culture media, quercetin, and Gallic acid were provided by Merck Co. (Darmstadt, Germany). Ciprofloxacin and gentamicin antibiotic discs were obtained from Paten Tab Co. (Tehran, Iran).
3.2. Preparation of the Plant Extracts
The colored and white skin of C. grandis were collected from Gilan Province, located in the north of Iran, in 2015 and extracted using soxhlet (12). Initially, 20 grams of dried powder was added to 200 milliliters of 80% methanol, 96% ethanol, and distilled water and shaken. Afterwards, the extracts were filtered and centrifuged at 10,000 rpm for eight minutes (13). The extracts were preserved in a dark glass bottle in the freezer at the temperature of -22°C (14).
3.3. Bacterial Strains
Gram-positive bacteria, such as Bacillus cereus (PTCC 1247), Streptococcus pyogenes (PTCC 1447), Bacillus subtilis (PTCC 1156), Micrococcus luteus (ATCC 10987), and Staphylococcus aureus (PTCC 1189), and Gram-negative bacteria, such as Salmonella typhi (PTCC 1609), Escherichia coli (ATCC 25922), Shigella boydii (PTCC 1141), Pseudomonas aeruginosa (PTCC 1181), and Enterobacter aerogenes (PTCC 1221) were provided by Hamadan University of Medical Sciences (Iran). To prepare a fresh bacterial culture, a bacterial colony was transferred to the solid MHA medium and incubated for 24 hours at the temperature of 37°C. The concentration of bacterial suspension was equivalent to 0.5 McFarland standard (1.5 × 108 CFU) (15).
3.4. Evaluation of Antibacterial Activity Using the Agar Well Diffusion Assay
At this stage, the concentration of 400 mg.mL-1 of 96% ethanol, 80% methanol, and aqueous extracts of the colored and white skin of C. grandis were prepared. Following that, 200 milliliters of the bacterial suspension (1.5 × 108 CFU) was poured onto the MHA medium. Wells with the diameter of five millimeters were prepared in Petri plates. Following that, 50 microliters (16) of the prepared concentration was poured into the wells and incubated at the temperature of 37°C for 24 hours (17). At the next stage, gentamicin (10 µg) and ciprofloxacin (0.005 µg) were used as the positive controls (3). The experiments were performed in triplicate, and data analysis was performed in SPSS version 16.
3.5. Determination of the MIC and MBC Using the Serial Dilution Method
To determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), 400 mg.mL-1 of 96% ethanol, 80% methanol, and the aqueous extracts of the white and colored skin extracts were used in the serial dilution method. The dilution series of 200, 100, 50, 25, 12.5, and 6.25 mg.mL-1 were prepared for the MIC assessment (18), and the one-second serial dilution was prepared. The broth nutrient culture medium with the non-bacterial extract was selected as the positive control, and the culture medium without the extract and containing the bacteria was used as the negative control. Following that, 20 microliters of the bacterial suspension (0.5 Mcfarland) was added to all the test tubes, except the positive control. The tubes were incubated for 24 hours at the temperature of 37°C. The lowest dilution of the extract with no bacterial growth was considered as the MIC. At this stage, five microliters of the tubes with no human bacterial growth was added to the MHA culture medium and incubated for 24 hours at the temperature of 37°C. The minimum concentration with no bacterial growth on the culture medium was considered as the MBC (14).
3.6. Determination of the Total Flavonoid and Phenolic Contents
The total flavonoid content was determined using the aluminum chloride method (19), with quercetin used as the standard. After 30 minutes at the temperature of 25°C, the absorption of the samples was measured at 415 nanometers using a spectrophotometer and determined as the milligram of quercetin per gram of the dry extract weight (mgQ/g). The total phenolic content was estimated using the Folin-Ciocalteu method (20), with Gallic acid used as the standard. The absorption of the samples was measured at 765 nanometers using a spectrophotometer after 15 minutes at the temperature of 25°C in darkness and determined as the milligram of Gallic acid per gram of the dry extract weight (mgGA/g).
3.7. Anti-radical Activity by the DPPH Assay
The free radical activity was investigated using the method proposed by Stojicevic et al. (21). The methanol extract of the colored and white skin of C. grandis were prepared at the concentrations of 0.2, 0.4, 0.6, 0.8, and 1 mg.mL-1, and ascorbic acid was used as the standard. Afterwards, the samples were placed in darkness for 30 minutes, and absorption was recorded using a spectrophotometer at 517 nanometers (22). The IC50 of the extracts and ascorbic acid were measured, and 99% methanol was used as the blank. The free radical scavenging activity (%) was calculated using the following formula:
RSA (%) = 100 (1 - (As - Ab)/Ac
where As shows the sample, Ab is the blank, and Ac represents the control.
3.8. Investigation of the Phytochemical Compounds
The methanol extract was used to determine the presence and absence of alkaloids, saponins, and tannins. To detect the presence of alkaloids, 0.5 gram of the methanol extract was dissolved in five milliliters of 1% HCl solution, preserved in warm distilled water for five minutes, filtered, and added the Mayer’s reagent. The sediment or turbidity indicated the presence of alkaloid (22, 23). To detect the presence of tannin, 0.5 gram of the methanol extract was dissolved in five milliliters of distilled water, filtered, and added to 10% FeCl3 chloride. The observation of the black-green color indicated the presence of tannin (22). To detect the presence of saponin, 20 milliliters of distilled water was added to 0.25 gram of the methanol extract, boiled, filtered, and shaken. The stable foam on the surface indicated the presence of saponin (22).
3.9. Statistical Analysis
Data analysis was performed in SPSS version 16, and the obtained results were expressed in a completely randomized design using a factorial test and average comparison by Duncan’s test at the significance level of P < 0.05.
4. Results
Table 1 shows the evaluation of the inhibitory effects of the alcoholic and aqueous extracts of C. grandis against human pathogenic bacteria, including the negative control (50 µL of used solvents) and positive controls (gentamicin and ciprofloxacin). After incubation, the bacterial growth inhibition zones were measured around the wells.
| Bacteria | Colored Skin | White Skin | Gentamicin | Ciprofloxacin | ||||
|---|---|---|---|---|---|---|---|---|
| Methanolaa, b | Ethanolba, b | Aqueousca, b | Methanolaa, b | Ethanolba, b | Aqueousca, b | |||
| B. subtilis | 13 ± 0.88M | 17.67 ± 0.33IJ | 13.33 ± 0.33ML | 14.5 ± 1.73KL | 11.67 ± 0.88NO | 9.33 ± 0.33PQ | 29 ± 0.57D | 29.5 ± 0.33D |
| B. cereus | 20 ± 0.58H | 17.33 ± 1.33IJ | 12.67 ± 1.2MN | 8.33 ± 0.88QR | 11 ± 1.53O | 9.67 ± 0.33PQ | 19.66 ± 0.33I | 28.5 ± 0.66D |
| S. aureus | 20.33 ± 0.33H | 16.67 ± 0.88J | 12.67 ± 0.33MN | 17.67 ± 0.33IJ | 15 ± 2.4K | 10 ± 0.58P | 20 ± 1H | 28.5 ± 0.66D |
| S. pyogenes | 8 ± 0.67R | 12.33 ± 0.33MN | 10 ± 1P | 16.8 ± 0.66J | 14.5 ± 0.00KL | 0 ± 0U | 20 ± 0.57H | 31.5 ± 0.33C |
| M. luteus | 20 ± 0.58H | 14 ± 0.58L | 9.33 ± 0.88PQ | 26.67 ± 1.76E | 13.67 ± 0.33ML | 0 ± 0.00U | 22 ± 0.33G | 30 ± 1C |
| S. typhi | 12 ± 0.58N | 12.67 ± 0.33MN | 13.33 ± 0.33ML | 13 ± 0.58M | 14 ± 1L | 15.67 ± 0.88JK | 29.5 ± 1D | 33 ± 0.57B |
| S. boydii | 11.67 ± 0.33NO | 7.67 ± 0.33S | 7.33 ± 0.33S | 13.33 ± 0.33ML | 10.33 ± 0.88OP | 0 ± 0.00U | 19 ± 0.57I | 37.5 ± 0.66A |
| P. aeruginosa | 5.33 ± 2.67T | 12 ± 0.58N | 0 ± 0U | 15.33 ± 1.2JK | 14.7 ± 0.67KL | 0 ± 0.00U | 20 ± 0.33H | 24.5 ± 0.66F |
| E. coli | 12.33 ± 0.33MN | 12.33 ± 0.33MN | 9 ± 0.33Q | 19.67 ± 0.88HI | 15.67 ± 1.2JK | 12.33 ± 0.33MN | 19.5 ± 1HI | 24.5 ± 0.57F |
| E. aerogenes | 14 ± 0.33l | 13 ± 0.58M | 12.33 ± 1.2MN | 22 ± 0.58G | 20.67 ± 0.88GH | 9.33 ± 1.33PQ | 11 ± 0.33O | 28 ± 0.33D |
Antibacterial Activity of Methanol, Ethanol, and Aqueous Extracts of White and Colored Skin of C. grandis Compared to Gentamicin and Ciprofloxacin
Table 1 also shows the findings regarding the inhibitory effects of the methanol, ethanol, and aqueous extracts of C. grandis (colored and white skin) on human infectious bacteria. In the colored skin, the methanol extract had more significant inhibitory effects compared to the ethanol and aqueous extracts. The highest diameter of the inhibition zone was observed in the methanol extract against B. cereus, S. aureus, and M. luteus. In addition, P. aeruginosa showed resistance to the aqueous extract. Compared to the gentamicin, the methanol extract showed had more significant inhibitory effects on B. cereus and E. aerogenes.
With regard to the inhibitory effects of the methanol, ethanol, and aqueous extracts of the white skin, S. pyogenes, S. boydii, M. luteus and P. aeruginosa showed resistance against the aqueous extract. On the other hand, the methanol extract had better inhibitory effects compared to the ethanol and aqueous extracts. The methanol extract showed the most significant growth inhibition zone in the case of M. luteus.
4.1. MIC and MBC
Table 2 shows the obtained results on the MIC and MBC of the colored and white skin extracts of C. grandis. Accordingly, the MIC (6.25 mg.mL-1) of the colored and white skin methanol extract was observed in B. cereus and S. aureus, while the inhibitory dilution (12.5 mg.mL-1) of the colored skin methanol extract was observed in S. aureus, M. luteus, and S. typhi. In the methanol extract of the white skin, such effect was observed on M. luteus and E. coli. However, the aqueous extract had no inhibitory effects on S. boydii and P. aeruginosa, while S. pyogenes and S. boydii showed resistance against all the tested dilutions.
| Organ | Con. | Bacteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | B. cereus | S. aureus | M. luteus | E. aerogenes | S. typhi | P. aeruginosa | E. coli | S. pyogenes | S. boydii | |||
| Colored skin | M | MIC | 25 | 6.25 | 12.5 | 12.5 | 50 | 12.5 | - | 50 | - | - |
| MBC | 50 | 50 | 50 | 100 | - | 25 | - | 100 | - | - | ||
| E | MIC | 50 | 25 | 25 | 50 | 25 | 25 | 100 | 25 | - | - | |
| MBC | - | 50 | 100 | 100 | 50 | 50 | - | 100 | - | - | ||
| A | MIC | 50 | 100 | 50 | - | 50 | 100 | - | - | - | - | |
| MBC | - | - | - | - | 100 | 200 | - | - | - | - | ||
| White skin | M | MIC | 25 | 50 | 6.25 | 12.5 | 50 | - | 100 | 12.5 | - | - |
| MBC | 50 | 100 | 25 | 50 | 100 | - | 100 | 50 | - | - | ||
| E | MIC | 50 | 25 | 25 | 25 | 25 | 50 | - | 50 | - | - | |
| MBC | 100 | 50 | 50 | 100 | 50 | 100 | - | - | - | - | ||
| A | MIC | - | - | 100 | 50 | - | 50 | - | 50 | - | - | |
| MBC | - | - | - | 100 | - | - | - | |||||
MIC and MBC (mg.mL-1) of Colored and White Skin Extracts of C. grandis Against Human Pathogenic Bacteria
4.2. Evaluation of Antioxidant Properties
4.2.1. Total Phenolic and Flavonoid Content
Table 3 shows the levels of flavonoids (mgQ/g) and phenols (mgGA/g) in the colored and white skin methanol extract of C. grandis. Accordingly, the total phenolic content of the colored and white skin was 79.71 and 71.63 mgGA/g, while the flavonoid content was estimated at 3.63 and 4.06 mgQ/g, respectively. Furthermore, a significant difference was observed in the total flavonoids between the colored and white skin extract at 5% probability level.
| Organ | Extract | Colored Skin | White Skin |
|---|---|---|---|
| Phenols, mgGA/g | Methanol | 79.71A | 71.63A |
| Flavonoids, mgQ/g | Methanol | 3.63B | 4.06A |
Total Phenolic and Flavonoid Contents of Colored and White Skin Methanol Extracts of C. grandisa
4.3. Anti-Radical Activity by DPPH
Table 4 shows the IC50 content. According to the results, the inhibitory effects of the DPPH free radicals increased at the higher concentrations of the extract. The IC50 of the colored and white skin methanol extract and ascorbic acid were estimated at 0.1251, 0.1376, and 0.1095 mg.mL-1, respectively. However, no significant difference was observed in the IC50 values between the methanol extract and ascorbic acid.
| Organ | Inhibition Rate of DPPH at Various Concentrations, mg.mL-1 | IC50 | ||||
|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | 1 | ||
| Colored skin | 79.92 | 86.13 | 93.81 | 96.4 | 98.47 | 0.1251A |
| White skin | 72.7 | 74.44 | 78.16 | 82.85 | 85.31 | 0.1376A |
| Ascorbic acid | 91.3 | 92.43 | 97.41 | 98.56 | 99.67 | 0.1095A |
Antioxidant Activity (IC50: mg.mL-1) of Colored and White Skin Methanol Extracts of C. grandis and Inhibition Rate of DPPH
4.4. Identification of Phytochemical Compounds
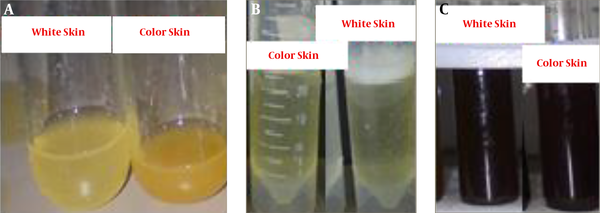
In this study, the methanol extract was used to confirm the presence or absence of secondary metabolites, including alkaloids, saponins, and tannins. The formation of sediment or turbidity indicated the presence of alkaloids (Figure 1A), the production of a stable foam showed the presence of saponins (Figure 1B), and the production of a black-green color represented the presence of tannins (Figure 1C).
Table 5 shows the presence or absence of secondary metabolites (alkaloids, saponins, and tannins). Accordingly, alkaloids were present and saponins and tannins were absent in the colored skin extract, while alkaloids and saponins were present and tannins were absent in the white skin extract.
| Organ | C. grandis | ||
|---|---|---|---|
| Alkaloid | Saponin | Tannin | |
| Colored skin | + | - | - |
| White skin | + | + | - |
Presence and Absence of Alkaloids, Saponins, and Tannins in Colored and White Skin Methanol Extracts of C. grandis
5. Discussion
Due to the increased bacterial resistance against antibiotics and side-effects of chemical drugs, the use of medicinal plants with antimicrobial properties has gained importance in disease treatment (15). According to the results of the present study, ethanol and methanol polar solvents could extract the compounds with antibacterial activity, such as aromatics, flavonoids, and flavonols, which were dissolved in the methanol and ethanol solvents (24, 25). In addition, the methanol extract of C. grandis exerted more significant inhibitory effects compared to the ethanol and aqueous extracts.
The literature search revealed that no prior studies have been focused on the antimicrobial activities of C. grandis in Iran. Meanwhile, the antimicrobial activity of ethyl acetate, butanol, and the methanol extracts of the white and colored skin of C. grandis has been investigated using the disc-diffusion method against S. aureus, B. cereus, B. subtilis, and E. coli (26). According to the findings, the methanol extract of the colored and white skin of C. grandis showed better inhibitory activities against B. cereus and S. aureus, which is consistent with our findings.
In a study in this regard, Mokbel and Suganuma investigated the antimicrobial activity of 80% and 100% methanol extract of C. grandis white skin against S. aureus, B. subtilis, B. cereus, M. luteus, and E. coli (5). According to the mentioned research, 80% methanol extract had more significant antibacterial effects compared to the pure methanol extract, and S. aureus had the highest susceptibility. In addition, the level of free radicals was estimated to be 74.5%, which in line with the present study. Several factors could affect antimicrobial activity, such as the extraction method, environmental conditions, plant genotype, moisture content, extraction time, and powder size (27). In the current research, the serial diffusion method yielded more accurate results compared to the disc-diffusion and agar well diffusion methods (28).
Citrus fruits contain high levels of phenolic compounds and ascorbic acid (29, 30). According to the findings of Gorinstein et al. (31), the flavonoid content of lemon, orange, and grape fruit is 1.9, 1.8, and 1.6 mgGA/g, respectively. The discrepancy between the mentioned study and our findings could be due to the differences in the species type, environmental conditions, and different compounds. In another research, Mathur et al. (32) reported the total phenol content of the skin ethanol extract of C. sinensis, C. maxima, and C. reticulata to be 0.13, 0.02, and 0.14 mgQ/g, respectively. Furthermore, Ghasemi et al. (33) reported the total flavonoid content of the skin methanol extract of 13 species from Iran to be within the range of 0.3 - 31.1 mgQ/g and the IC50 content to be within the range of 0.6 - 2.9 mg/mL; the IC50 content obtained in the mentioned study is consistent with our findings. On the same note, Fatahimoghadam et al. (34) reported the total flavonoid content of the skin methanol extract of six citrus fruits from the north of Iran to be within the range of 2.65 - 7.68 mgQ/g and the free radical inhibition percentage within the range of 68.58 - 95.77%, which is consistent with the results of the present study.
Considering the higher solubility of active compounds with antimicrobial properties in methanol solvents (35), we used the methanol extract to detect the presence or absence of secondary metabolites. In this regard, Tian-Shung et al. (36) assessed the presence of alkaloids by an acetone extract, and Okwu et al. (37) evaluated the presence of tannins, alkaloids, and saponins by the diethyl ether extract of C. grandis skin; the results of the mentioned studies are consistent with our findings. In another study, Mishra et al. (38) detected the presence of tannins and saponins by the methanol extract of C. limetta skin using a phytochemical method. In addition, Sheikhlar et al. (39) reported the presence of alkaloids (consistent with our findings) and tannins, as well as the absence of saponins (consistent with our findings in the colored skin extract) by the skin methanol extract of C. limon using a phytochemical method. Similarly, Pandey et al. (16) reported the presence of tannins (contrary to our findings) and absence of saponins by the skin and seed methanol extract of C. limon. Pathan et al. (40) also reported the presence of tannins and alkaloids by the leaf and skin hydro-alcoholic extract of C. aurantium. The difference in the presence and absence of secondary metabolites in various plants depends on the variations in the species type, extract and solvent, environmental conditions, and extraction methods (41).
5.1. Conclusions
According to the results, the extract of C. grandis had antibacterial and antioxidant activities owing to the presence of secondary metabolites. Therefore, it is recommended that by the processing and extraction of antimicrobial compounds from the extract of this plant, C. grandis be used in the pharmaceutical industry as an antibiotic to control human pathogenic bacteria.