1. Background
Oral infections are common health conditions that are caused by various microorganisms, especially the normal flora of the oral cavity. These infections could spread to other body organs (e.g., heart and lungs) and cause systemic diseases and infective endocarditis. Viridans streptococci (especially Streptococcus mutans) are known as the pioneer microorganisms that initiate oral biofilm and dental plaque formation in humans (1, 2).
Biofilms are communities of densely packed bacteria, embedded within a slimy extracellular polymeric substance produced by these microorganisms, which is mainly comprised of polysaccharides and other biomolecules, such as proteins, lipids, and nucleic acids (3). These complex systems contain a high density of bacterial cells (108 - 1011 bacterial cells/gram) of various species (4). Dental plaque is a spatially structured biofilm, which is composed of a complex microbial community (5). The microbial components of dental plaque are bacterial components such as glycosyltransferases and glucans. S. mutans is an important bacterium that colonizes on tooth surfaces. The negative charge of the bacterial cell wall facilitates attachment to the positively charged receptor molecules on the polyclonal surface (6).
Considering the inadvertent side-effects of the chemicals used in commercially available mouthwashes and growing incidence of drug-resistant oral infections (7, 8), the use of natural compounds (especially medicinal herbs) for the prevention, control, and treatment of tooth decay has been suggested as a promising approach. Oregano (Origanum vulgare) is a plant native to Iran, which is mainly cultivated in Guilan, Mazandaran, and Azerbaijan provinces (9). Oregano essential oil is an abundant source of potent antioxidants and antimicrobial components (10).
2. Objectives
As in vivo studies provide more information for practical solutions, the present study aimed to evaluate the role of streptococcal biofilms in the formation of dental plaque and dental caries and investigate the in vitro and in vivo anti-biofilm effects of oregano essential oil.
3. Methods
3.1. Patients and Bacterial Isolation
This experimental study was conducted on 150 samples obtained from the buccal and lingual surfaces of the posterior teeth of elementary school students aged 7 - 12 years with the same gender ratio in Gorgan, located in the north of Iran. The samples were collected using a sterile swab and dental floss. The students who used antibiotics within the past three months or had systemic or immunodeficiency disorders were excluded from the study.
The study procedures were performed in accordance with medical ethics standards. The samples were collected and transferred to the laboratory in sterile tubes containing the brain heart infusion (BHI) broth (Merck, Germany) and buffer. After two hours of anaerobic incubation at the temperature of 37°C, the samples were homogenized by vortexing and cultured on blood agar (Merck, Germany) containing 7% defibrinated sheep blood for 24 hours at the temperature of 37°C in anaerobic conditions. Following that, S. mutans was identified based on the colony morphology, gram staining, biochemical tests (hemolysis, catalase, bile esculin, optochin susceptibility, methyl red/Voges-Proskauer, and arginine dihydrolase), and fermentation of mannitol, lactose, salicin, and trehalose. The standard strain of S. mutans PTCC35668 was also used as the control.
3.2. Preparation of the Oregano Essential Oil and MIC Determination
Oregano (Origanum vulgare) was collected from the mountains around Gorgan city in the noetheast of Iran. After the extraction of the oregano leaves, they were mixed, soacked in 70% ethanol at the ratio of 1:10 (each 100 cc of essence soaked in 1,000 cc of ethanol), and placed on a shaker. After 24 hours, the mixture was filtered and placed in a rotary evaporator to evaporate the solvent (ethanol). The maceration method was used to prepare the aqueous essential oil. For this purpose, the filtrate was heated for 15 minutes without boiling, and the mixture was preserved in a container at room temperature to cool. Afterwards, the mixture was filtered through a Whatman no.: 2 filter paper (USA) and stored in a glass container at the temperature of 4°C until use. In addition, a stock solution was prepared from the essential oil of oregano in dimethyl sulfoxide within the concentration range of 4 - 2,048 µL/mL.
The minimum inhibitory concentration (MIC) of the oregano essential oil on the S. mutans isolates was determined using the broth microdilution method. Initially, the serial dilutions were prepared by inoculating 50 microliters of the oregano essential oil into the wells of a 96-well microplate containing 50 microliters of the Mueller Hinton broth. Following that, 50 microliters of S. mutans suspension (0.5 McFarland standard) was inoculated into each well of the microplate. After incubation in anaerobic conditions, the MIC of the essential oil was determined by reading the absorbance at 630 nanometers using an ELISA reader (BioTec, Germany).
3.3. Identification of the Oregano Essential Oil Components
Gas chromatography–mass spectrometry (GC-MS) was used for the identification of the antimicrobial compounds in the oregano essential oil. After the injection of the oregano essential oil into a gas chromatograph and determining the optimal column temperature, the essential oil was diluted with dichloromethane and injected into a GC-MS instrument. Finally, the constituents of the essential oil were determined quantitatively and qualitatively by analyzing the obtained mass spectra, as well as the corresponding chromatograms based on the retention time, Kovats retention index, and comparison of the mass spectra with the standard compounds using the Saturn GC/MS Workstation.
3.4. Biofilm Formation
Biofilm formation by the S. mutans isolates was evaluated using the microtiter plate method. A bacterial suspension equivalent to 0.5 McFarland standard (optical density [OD] of 0.08 - 1 at 625 nm) was prepared in tryptic soy broth (Merck, Germany). To each ELISA well, 100 microliters of the medium was added, along with 50 microliters of the bacterial suspension and 50 microliters of distilled water. The wells containing only the medium were considered as the negative controls. At the next stage, the microplate was incubated at the temperature of 37°C in 5% CO2 for 24 hours. After discarding the supernatant and washing the wells with phosphate buffered saline, the plate was shaken vigourosly in order to remove the unattached bacteria, and the attached bacteria were fixed with 96% ethanol and dried at room temperature. After staining with 2% crystal violet, the plate was examined for the presence of ring-shaped purple stains. Biofilm formation was analyzed by adding 200 microliters of 33% glacial acetic acid to each well and measuring OD at 492 nanometers using an ELISA reader. The OD of lower than 0.1 indicated the absence of biofilm formation, the OD of 0.1 - 0.2 showed weak biofilm formation, the values of 0.2 - 0.3 indicated moderate biofilm formation, and higher OD values than 0.3 showed potent biofilm formation.
3.5. Anti-biofilm Effects of the Oregano Essential Oil
The anti-biofilm effects of the oregano essential oil were evaluated using the modified microtiter plate method. For this purpose, 100 microliters of tryptic soy broth containing 2% dextran was added to the wells of the 96-well microplate. Afterwards, 50 microliters of various concentrations of the oregano essential oil and 50 microliters of the S. mutans suspension were added to each well. The wells containing 200 microliters of the culture medium were considered as the negative controls. The microplate was incubated at the temperature of 37°C for 24 hours, and the bacteria were fixed and stained as mentioned earlier. All the experiments were performed in triplicate.
3.6. In Vivo Experiments
In this study, 18 male NMRI mice with the approximate weight of 30 - 40 grams were purchased from the Pasteur Institute of Iran, Amol branch. The animals were kept in three separate cages at the temperature of 22 ± 3°C within a 12-hour light/dark cycle and had access to food and water ad libitum. The mice were allowed to adapt to the ambient conditions for two weeks. Following that, they were divided into two groups of case (n = 9) and control (n = 9) in order to confirm the biofilm formation by the S. mutans isolates and assess the potential anti-biofilm properties of the oregano essential oil. To suppress the bacterial flora of the oral cavity, the animals received an oral suspension of water and penicillin G (600 units/mL) for four days. In both groups, the suspension of biofilm-producing S. mutans isolates (turbidity: 3 McFarland standard) was rubbed on the animals' teeth using sterile swabs twice per day, along with a sweet diet for 40 days. In the control group, the applied samples were identical to the case group (concentrations equal to the bacterial suspension) with the oregano essential oil (2,048 µL/mL).
In this study, all the tests were performed at a specified time, and the development of dental caries was tracked using a magnifying glass. In addition, the presence of S. mutans in the swab samples obtained from the dental plaques was investigated by gram staining and erythrosine.
3.7. Statistical Analysis
Data analysis was performed in SPSS version 23 using the Kolmogorov-Smirnov test, Kruskal-Wallis test, and one-way analysis of variance (ANOVA) at the significance level of 0.05.
4. Results
S. mutans were identified in 23 samples (15.3%). The frequency of the bacteria was 52% and 48% in girls and boys, respectively, and no significant correlation was observed between the age of the subjects and positivity in this regard.
4.1. Results of the Broth Microdilution Assay
Our evaluations revealed that 52.2% of the isolates did not grow after the treatment with the oregano essential oil at the concentration of 512 µL/mL. The mean MIC of the oregano essential oil was 50 µl/ml against the S. mutans isolates and 128 µL/mL against the standard strain. Furthermore, the oregano essential oil could inhibit 90% of the S. mutans isolates at the concentration of 2,048 µL/mL (MIC90: 2,048 µL/mL).
4.2. Results of the GC-MS Analysis of the Oregano Essential Oil Constituents
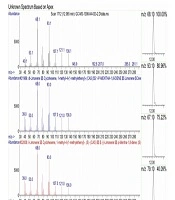
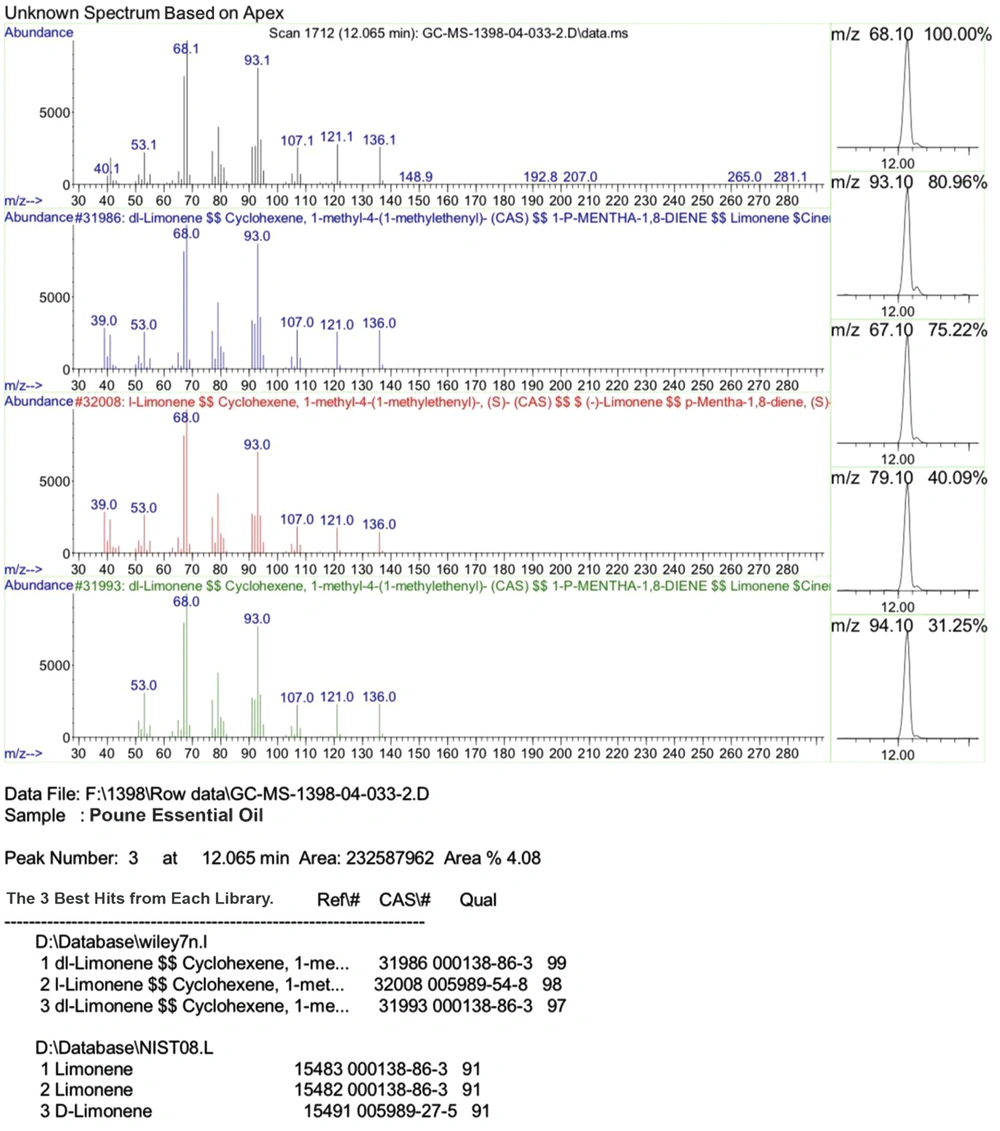
Among the identified compounds in the oregano essential oil, limonene and myrcene appeared at the retention time of 12.065 and 10.812, respectively. In addition, limonene and myrcene constituted 4.08% and 0.33% of the total content of the essential oil, respectively (Figure 1).
4.3. Results of the Biofilm Production and Anti-biofilm Properties of the Oregano Essential Oil
Among the S. mutans isolates (n = 23), 20 isolates (87%) were capable of biofilm formation. According to the information in Table 1, the treatment of the isolates with the MIC and sub-MIC concentrations of the oregano essential oil could inhibit the biofilm formation ability of the isolates by 80% - 85%.
| Biofilm Formation Ability of Untreated Isolates | Treatment of Isolates with Oregano Essential Oil | ||
|---|---|---|---|
| MIC | Sub-MIC | ||
| Potent biofilm | 3 (15) | ||
| No Biofilm | 2 (66.7) | 1 (33.3) | |
| Weak Biofilm | 1 (33.3) | 2 (66.7) | |
| Moderate biofilm | 11 (55) | ||
| No Biofilm | 10 (90.1) | 8 (72.7) | |
| Weak Biofilm | 1 (9.1) | 3 (27.3) | |
| Weak biofilm | 6 (30) | ||
| No Biofilm | 6 (100) | 6 (100) | |
| Weak Biofilm | 0 | 0 | |
| Total 20 | (100) | ||
| No Biofilm | 17 (85) | 16 (80) | |
| Weak Biofilm | 3 (15) | 4 (20) | |
aValues are expressed as No. (%).
4.4. Results of the In Vivo Investigations
The results of the Kruskal-Wallis test indicated a significant correlation between treatment with the oregano essential oil and biofilm formation by the S. mutans isolates (P = 0.05) (Figure 2). In fact, no tooth decay was observed in the mice treated with the essential oil of oregano.
5. Discussion
S. mutans is the most common cause of tooth decay (11). According to epidemiological studies, the prevalence of these bacteria may vary across countries and ethnicities (12). In the present study, the prevalence of S. mutans was estimated at 15%, which was lower compared to the other studies conducted in Iran (8), Brazil (85.7%) (13), and Iraq (71.4%) (14). The discrepancy could be due to environmental and individual factors such as dietary habits, fluoride use, oral hygiene, salivary flow, immunological parameters, and knowledge improvement (15).
According to the World Health Organization (WHO), tooth decay remains a global health concern that affects both children and adults, especially those with a poor socioeconomic status. Considering the high rate of drug resistance in the bacteria that cause tooth decay, prevention by inhibiting biofilm formation seems to be a more rational solution to this health issue (8, 16).
In the present study, 87.3% of the S. mutans isolates were capable of biofilm formation. In a previous study performed in Iran in 2013, the frequency of biofilm-forming S. mutans was reported to be 65% among the children aged 4 - 6 years old, which is lower compared to the estimated rate in the present study. This discrepancy could be due to the difference in the age of the subjects and variability of the adhesion mediators of the bacteria. Some researchers have also demonstrated the ability of salivary agglutinin and sortase A in biofilm formation by S. mutans (17, 18).
Considering the advantages of medicinal plants over chemical drugs (e.g., cost-effectiveness and lack of side-effects), we evaluated the anti-biofilm properties of the essential oil of oregano in the current research. A similar study indicated that the ethanolic extract of bell pepper exerted relatively favorable antimicrobial effects against S. mutans (19). Moreover, two other studies conducted in Iran have confirmed the bactericidal and anti-biofilm properties of black tea (20) and Chlorella vulgaris extracts (21).
The MIC refers to the lowest concentration of a compound that is able to inhibit the growth of a microorganism after a specified incubation period (16 - 24 hours depending on the bacterial species) (22). In the present study, the MIC of the oregano essential oil against the S. mutans isolates was 512 µL/mL. Previous studies have also reported the potent antibacterial and anti-biofilm effects of oregano essential oil on other bacterial species (23, 24).
According to the GC-MS analysis in the current research, the main constituents of the oregano essential oil were limonene and myrcene. In the previous studies in this regard, the antimicrobial and antimicrobial properties of this essential oil have mainly been attributed to carvacrol and thymol (22, 25). Our in vivo investigation also confirmed the potent antibacterial and anti-biofilm properties of the oregano essential oil. Furthermore, our findings suggested that mice (especially young mice) are a proper model for assessing and tracking dental caries owing to their small size and favorable maintenance conditions.
5.1. Conclusion
The measurement of species abundance could be used to asses and track the progress of tooth decay in research alone. It has also been shown that biofilm formation on the tooth surface leads to oral and dental diseases depending on the major determinants of bacterial pathogenicity, such as the type and proportion of the bacterial population. Considering the favorable anti-biofilm and antibacterial properties of limonene and myrcene, these compounds could be further investigated for the production of plant-based anti-tooth decay and oral mouthwashes.